Vitamin C—An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19
Abstract
:1. Introduction
2. Vitamin C Deficiency in Pneumonia, Sepsis and COVID-19
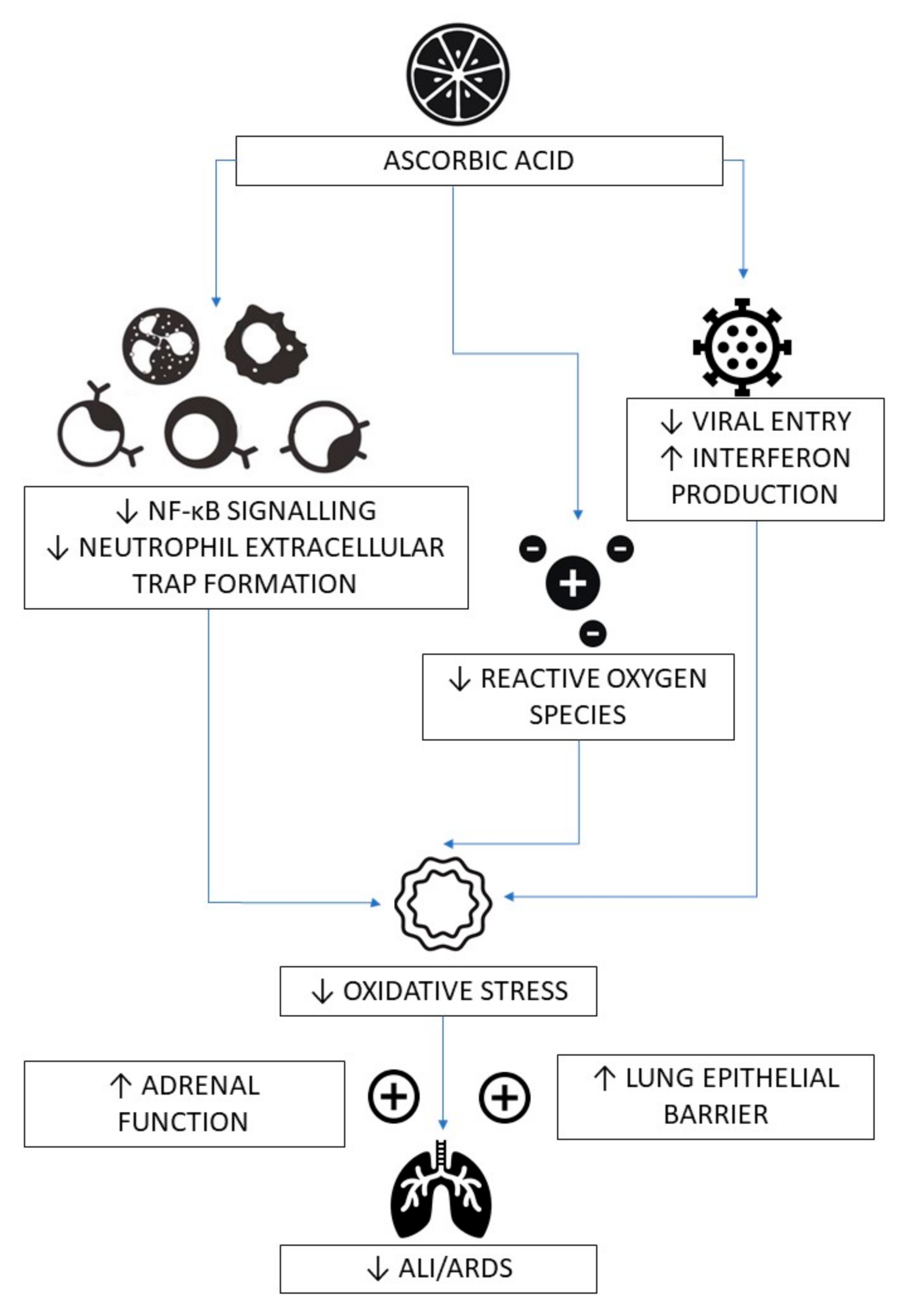
3. Mechanisms of Action of Vitamin C in Infections, Sepsis and COVID-19
4. Clinical Evidence for the Role of Vitamin C in Colds
5. Clinical Evidence for the Role of Vitamin C in Pneumonia
6. Clinical Evidence for the Role of Vitamin C in Critically Ill Septic Patients
7. Clinical Evidence for the Role of Vitamin C in COVID-19
8. Safety of Oral and Intravenous Vitamin C
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drouin, G.; Godin, J.R.; Page, B. The genetics of vitamin C loss in vertebrates. Curr. Genomics 2011, 12, 371–378. [Google Scholar] [CrossRef]
- Milton, K. Micronutrient intakes of wild primates: Are humans different? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 136, 47–59. [Google Scholar] [CrossRef]
- Milton, K. Nutritional characteristics of wild primate foods: Do the diets of our closest living relatives have lessons for us? Nutrition 1999, 15, 488–498. [Google Scholar] [CrossRef]
- European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on dietary reference values for vitamin C. EFSA J. 2013, 11, 3418. [Google Scholar] [CrossRef] [Green Version]
- Bates, B.; Collins, D.; Cox, L.; Nicholson, S.; Page, P.; Roberts, C.; Steer, T.; Swan, G. National Diet and Nutrition Survey Years 1 to 9 of the Rolling Programme (2008/2009–2016/2017): Time Trend and Income Analyses; Public Health England: London, UK, 2019. [Google Scholar]
- Berger, M.M.; Bischoff-Ferrari, H.A.; Zimmermann, M.; Herter, I.; Spieldenner, J.; Eggersdorfer, M. White Paper on Nutritional Status in Supporting a Well-Functioning Immune System for Optimal Health with a Recommendation for Switzerland; SGE: Bern, Switzerland, 2020. [Google Scholar]
- Linus Pauling Institute; Micronutrient Information Center. Micronutrients for Older Adults Oregon State University. 2020. Available online: https://lpi.oregonstate.edu/mic/life-stages/older-adults (accessed on 20 October 2020).
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, M.; Wang, Y.; Padayatty, S.J.; Morrow, J. A new recommended dietary allowance of vitamin C for healthy young women. Proc. Natl. Acad. Sci. USA 2001, 98, 9842–9846. [Google Scholar] [CrossRef] [Green Version]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef]
- De Grooth, H.J.; Manubulu-Choo, W.P.; Zandvliet, A.S.; Spoelstra-de Man, A.M.E.; Girbes, A.R.; Swart, E.L.; Oudemans-van Straaten, H.M. Vitamin-C pharmacokinetics in critically ill patients: A randomized trial of four intravenous regimens. Chest 2018, 153, 1368–1377. [Google Scholar] [CrossRef]
- Hume, R.; Weyers, E. Changes in leucocyte ascorbic acid during the common cold. Scott. Med. J. 1973, 18, 3–7. [Google Scholar] [CrossRef]
- Evans-Olders, R.; Eintracht, S.; Hoffer, L.J. Metabolic origin of hypovitaminosis C in acutely hospitalized patients. Nutrition 2010, 26, 1070–1074. [Google Scholar] [CrossRef]
- Teixeira, A.; Carrie, A.S.; Genereau, T.; Herson, S.; Cherin, P. Vitamin C deficiency in elderly hospitalized patients. Am. J. Med. 2001, 111, 502. [Google Scholar] [CrossRef]
- Fain, O.; Paries, J.; Jacquart, B.; Le Moel, G.; Kettaneh, A.; Stirnemann, J.; Heron, C.; Sitbon, M.; Taleb, C.; Letellier, E.; et al. Hypovitaminosis C in hospitalized patients. Eur. J. Intern. Med. 2003, 14, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.; Eintracht, S.; Hoffer, L.J. Vitamin C deficiency in a university teaching hospital. J. Am. Coll. Nutr. 2008, 27, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, P.; Wiltshire, S.; Das, K.; Wilson, R.B. Vitamin C deficiency in an Australian cohort of metropolitan surgical patients. Pathology 2018, 50, 654–658. [Google Scholar] [CrossRef]
- Hunt, C.; Chakravorty, N.K.; Annan, G.; Habibzadeh, N.; Schorah, C.J. The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. Int. J. Vitam. Nutr. Res. 1994, 64, 212–219. [Google Scholar] [PubMed]
- Bates, C.J.; Prentice, A.; Cole, T.J.; van der Pols, J.C.; Doyle, W.; Finch, S.; Smithers, G.; Clarke, P.C. Micronutrients: Highlights and research challenges from the 1994-5 National Diet and Nutrition Survey of people aged 65 years and over. Br. J. Nutr. 1999, 82, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemilä, H.; Louhiala, P. Vitamin C may affect lung infections. J. R. Soc. Med. 2007, 100, 495–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myint, P.K.; Wilson, A.M.; Clark, A.B.; Luben, R.N.; Wareham, N.J.; Khaw, K.T. Plasma vitamin C concentrations and risk of incident respiratory diseases and mortality in the European Prospective Investigation into Cancer-Norfolk population-based cohort study. Eur. J. Clin. Nutr. 2019, 73, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H.; Louhiala, P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [Green Version]
- Kory, P.; Kanne, J.P. SARS-CoV-2 organising pneumonia: ‘Has there been a widespread failure to identify and treat this prevalent condition in COVID-19?’. BMJ Open Respir. Res. 2020, 7, e000724. [Google Scholar] [CrossRef]
- Carr, A.C.; Spencer, E.; Dixon, L.; Chambers, S.T. Patients with community acquired pneumonia exhibit depleted vitamin C status and elevated oxidative stress. Nutrients 2020, 12, 1318. [Google Scholar] [CrossRef]
- Bakaev, V.V.; Duntau, A.P. Ascorbic acid in blood serum of patients with pulmonary tuberculosis and pneumonia. Int. J. Tuberc. Lung. Dis. 2004, 8, 263–266. [Google Scholar]
- Chakrabarti, B.; Banerjee, S. Dehydroascorbic acid level in blood of patients suffering from various infectious diseases. Proc. Soc. Exp. Biol. Med. 1955, 88, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Mochalkin, N.I. Ascorbic acid in the complex therapy of acute pneumonia. Voen. Med. Zhurnal 1970, 9, 17–21. [Google Scholar]
- Fowler, A.A., III; Truwit, J.D.; Hite, R.D.; Morris, P.E.; DeWilde, C.; Priday, A.; Fisher, B.; Thacker, L.R., II; Natarajan, R.; Brophy, D.F.; et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: The CITRIS-ALI randomized clinical trial. JAMA 2019, 322, 1261–1270. [Google Scholar] [CrossRef]
- Carr, A.C.; Rosengrave, P.C.; Bayer, S.; Chambers, S.; Mehrtens, J.; Shaw, G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care 2017, 21, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, A.A.; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; DeWilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014, 12, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doise, J.M.; Aho, L.S.; Quenot, J.P.; Guilland, J.C.; Zeller, M.; Vergely, C.; Aube, H.; Blettery, B.; Rochette, L. Plasma antioxidant status in septic critically ill patients: A decrease over time. Fundam. Clin. Pharmacol. 2008, 22, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Voigt, K.; Kontush, A.; Stuerenburg, H.-J.; Muench-Harrach, D.; Hansen, H.C.; Kunze, K. Decreased plasma and cerebrospinal fluid ascorbate levels in patients with septic encephalopathy. Free Radic. Res. 2002, 36, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Schorah, C.J.; Downing, C.; Piripitsi, A.; Gallivan, L.; Al-Hazaa, A.H.; Sanderson, M.J.; Bodenham, A. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am. J. Clin. Nutr. 1996, 63, 760–765. [Google Scholar] [CrossRef]
- Arvinte, C.; Singh, M.; Marik, P.E. Serum levels of vitamin C and vitamin D in a cohort of critically ill COVID-19 patients of a north American community hospital intensive care unit in May 2020: A pilot study. Med. Drug Discov. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chiscano-Camón, L.; Ruiz-Rodriguez, J.C.; Ruiz-Sanmartin, A.; Roca, O.; Ferrer, R. Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit. Care 2020, 24, 522. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C. Vitamin C in pneumonia and sepsis. In Vitamin C: New Biochemical and Functional Insights; Chen, Q., Vissers, M., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2020; pp. 115–135. [Google Scholar]
- Marik, P.E.; Hooper, M.H. Doctor-your septic patients have scurvy! Crit. Care 2018, 22, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marik, P.E. Vitamin C: An essential “stress hormone” during sepsis. J. Thorac. Dis. 2020, 12, S84–S88. [Google Scholar] [CrossRef]
- Marik, P.E. Vitamin C for the treatment of sepsis: The scientific rationale. Pharmacol. Ther. 2018, 189, 63–70. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Marik, P.E. The antiviral properties of vitamin C. Expert Rev. Anti Infect. Ther. 2020, 18, 99–101. [Google Scholar] [CrossRef]
- Thomas, W.R.; Holt, P.G. Vitamin C and immunity: An assessment of the evidence. Clin. Exp. Immunol. 1978, 32, 370–379. [Google Scholar]
- Dahl, H.; Degre, M. The effect of ascorbic acid on production of human interferon and the antiviral activity in vitro. Acta Pathol. Microbiol. Scand. B 1976, 84, 280–284. [Google Scholar] [CrossRef]
- Webb, A.L.; Villamor, E. Update: Effects of antioxidant and non-antioxidant vitamin supplementation on immune function. Nutr. Rev. 2007, 65, 181–217. [Google Scholar] [CrossRef]
- Hemila, H. Vitamin C and infectious diseases. In Vitamin C; Paoletti, R., Sies, H., Bug, J., Grossi, E., Poli, A., Eds.; Springer: Milan, Italy, 1998; pp. 73–85. [Google Scholar]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Russo, T.A.; Kwon, O.; Chanock, S.; Rumsey, S.C.; Levine, M. Ascorbate recycling in human neutrophils: Induction by bacteria. Proc. Natl. Acad. Sci. USA 1997, 94, 13816–13819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nualart, F.J.; Rivas, C.I.; Montecinos, V.P.; Godoy, A.S.; Guaiquil, V.H.; Golde, D.W.; Vera, J.C. Recycling of vitamin C by a bystander effect. J. Biol. Chem. 2003, 278, 10128–10133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, J.M.; Qu, Z.C. Ascorbic acid prevents oxidant-induced increases in endothelial permeability. Biofactors 2011, 37, 46–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, J.M.; Harrison, F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox Signal 2013, 19, 2068–2083. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.K.; Packer, L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996, 10, 709–720. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, G.; Yuan, J.; Wang, Y.; Yang, X.; Wang, X.; Li, G.; Liu, Z.; Zhong, N. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediators Inflamm. 2014, 2014, 426740. [Google Scholar] [CrossRef] [Green Version]
- Erol, N.; Saglam, L.; Saglam, Y.S.; Erol, H.S.; Altun, S.; Aktas, M.S.; Halici, M.B. The protection potential of antioxidant vitamins against acute respiratory distress syndrome: A rat trial. Inflammation 2019, 42, 1585–1594. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020, 181, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.; Bae, S.; Choi, J.; Lim, S.Y.; Lee, N.; Kong, J.M.; Hwang, Y.I.; Kang, J.S.; Lee, W.J. Vitamin C is an essential factor on the anti-viral immune responses through the production of interferon-a/b at the initial stage of influenza A virus (H3N2) infection. Immune Netw. 2013, 13, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Hemilä, H. Vitamin C and infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef] [Green Version]
- Atherton, J.G.; Kratzing, C.C.; Fisher, A. The effect of ascorbic acid on infection chick-embryo ciliated tracheal organ cultures by coronavirus. Arch. Virol. 1978, 56, 195–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davelaar, F.G.; Bos, J. Ascorbic acid and infectious bronchitis infections in broilers. Avian Pathol. 1992, 21, 581–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, R.; Rosoman, N.P.; Henshaw, D.J.E.; Noble, E.P.; Georgius, P.; Sommerfeld, N. COVID-19 as a viral functional ACE2 deficiency disorder with ACE2 related multi-organ disease. Med. Hypotheses 2020, 144, 110024. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24. [Google Scholar]
- Ma, S.; Sun, S.; Li, J.; Fan, Y.; Qu, J.; Sun, L.; Wang, S.; Zhang, Y.; Yang, S.; Liu, Z.; et al. Single-cell transcriptomic atlas of primate cardiopulmonary aging. Cell Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Jena, M. In silico virtual screening-based study of nutraceuticals predicts the therapeutic potentials of folic acid and its derivatives against COVID-19. Res. Square 2020. [Google Scholar] [CrossRef]
- Bosmann, M.; Ward, P.A. The inflammatory response in sepsis. Trends Immunol. 2013, 34, 129–136. [Google Scholar] [CrossRef]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Farkas, D.; Brophy, D.F.; Fowler, A.A., III; Natarajan, R. Vitamin C: A novel regulator of neutrophil extracellular trap formation. Nutrients 2013, 5, 3131–3151. [Google Scholar] [CrossRef] [Green Version]
- Fisher, B.J.; Kraskauskas, D.; Martin, E.J.; Farkas, D.; Wegelin, J.A.; Brophy, D.; Ward, K.R.; Voelkel, N.F.; Fowler, A.A., III; Natarajan, R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L20–L32. [Google Scholar] [CrossRef] [PubMed]
- Hornig, D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann. N. Y. Acad. Sci. 1975, 258, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Doppman, J.L.; Chang, R.; Wang, Y.; Gill, J.; Papanicolaou, D.A.; Levine, M. Human adrenal glands secrete vitamin C in response to adrenocorticotrophic hormone. Am. J. Clin. Nutr. 2007, 86, 145–149. [Google Scholar] [CrossRef]
- Kodama, M.; Kodama, T.; Murakami, M.; Kodama, M. Vitamin C infusion treatment enhances cortisol production of the adrenal via the pituitary ACTH route. In Vivo 1994, 8, 1079–1085. [Google Scholar] [PubMed]
- Barabutis, N.; Khangoora, V.; Marik, P.E.; Catravas, J.D. Hydrocortisone and ascorbic acid synergistically prevent and repair lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Chest 2017, 152, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Recovery Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in hospitalized patients with Covid-19-preliminary report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. The significance of the evidence about ascorbic acid and the common cold. Proc. Natl. Acad. Sci. USA 1971, 68, 2678–2681. [Google Scholar] [CrossRef] [Green Version]
- Pauling, L. Vitamin C the Common Cold and Flu; Freeman: San Francisco, CA, USA, 1970. [Google Scholar]
- Hemilä, H.; Chalker, E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Straten, M.; Josling, P. Preventing the common cold with a vitamin C supplement: A double-blind, placebo-controlled survey. Adv. Ther. 2002, 19, 151–159. [Google Scholar] [CrossRef]
- Klenner, F.R. Massive doses of vitamin C and the virus diseases. South Med. Surg. 1951, 113, 101–107. [Google Scholar]
- Pitt, H.A.; Costrini, A.M. Vitamin C prophylaxis in marine recruits. JAMA 1979, 241, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Kimbarowski, J.A.; Mokrow, N.J. Colored precipitation reaction of the urine according to Kimbarowski (FARK) as an index of the effect of ascorbic acid during treatment of viral influenza. Dtsch Gesundheitsw. 1967, 22, 2413–2418. [Google Scholar] [PubMed]
- Glazebrook, A.J.; Thomson, S. The administration of vitamin C in a large institution and its effect on general health and resistance to infection. J. Hyg. 1942, 42, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Nabil Habib, T.; Ahmed, I. Early adjuvant intravenous vitamin C treatment in septic shock may resolve the vasopressor dependence. Int. J. Microbiol. Adv. Immunol. 2017, 5, 77–81. [Google Scholar]
- Zabet, M.H.; Mohammadi, M.; Ramezani, M.; Khalili, H. Effect of high-dose ascorbic acid on vasopressor’s requirement in septic shock. J. Res. Pharm. Pract. 2016, 5, 94–100. [Google Scholar]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; De Backer, D.; Xiang, H.; et al. High-dose vitamin C infusion for the treatment of critically ill COVID-19. Res. Square 2020. [Google Scholar] [CrossRef]
- Zhang, M.; Jativa, D.F. Vitamin C supplementation in the critically ill: A systematic review and meta-analysis. SAGE Open Med. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Hemila, H.; Chalker, E. Vitamin C can shorten the length of stay in the ICU: A meta-analysis. Nutrients 2019, 11, 708. [Google Scholar] [CrossRef] [Green Version]
- Hemila, H.; Chalker, E. Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: A meta-regression analysis. J. Intensive Care 2020, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Long, C.L.; Maull, K.I.; Krishnan, R.S.; Laws, H.L.; Geiger, J.W.; Borghesi, L.; Franks, W.; Lawson, T.C.; Sauberlich, H.E. Ascorbic acid dynamics in the seriously ill and injured. J. Surg. Res. 2003, 109, 144–148. [Google Scholar] [CrossRef]
- Kashiouris, M.G.; L’Heureux, M.; Cable, C.A.; Fisher, B.J.; Leichtle, S.W.; Fowler, A.A. The emerging role of vitamin C as a treatment for sepsis. Nutrients 2020, 12, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marik, P.E.; Kory, P.; Varon, J.; Iglesias, J.; Meduri, G.U. MATH+ protocol for the treatment of SARS-CoV-2 infection: The scientific rationale. Expert Rev. Anti Infect. Ther. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H.; Chalker, E. Reanalysis of the effect of vitamin C on mortality in the CITRIS-ALI trial: Important findings dismissed in the trial report. Front. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.A., III; Fisher, B.J.; Kashiouris, M.G. Vitamin C for sepsis and acute respiratory failure—Reply. JAMA 2020, 323, 792–793. [Google Scholar] [CrossRef]
- Fujii, T.; Luethi, N.; Young, P.J.; Frei, D.R.; Eastwood, G.M.; French, C.J.; Deane, A.M.; Shehabi, Y.; Hajjar, L.A.; Oliveira, G.; et al. Effect of Vitamin C, Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients with Septic Shock: The VITAMINS Randomized Clinical Trial. JAMA 2020, 323, 423–431. [Google Scholar] [CrossRef]
- Long, M.T.; Kory, P.; Marik, P. Vitamin C, hydrocortisone, and thiamine for septic shock. JAMA 2020, 323, 2203–2204. [Google Scholar] [CrossRef]
- Carr, A.C. Is the VITAMINS RCT indicating potential redundancy between corticosteroids and vitamin C? Crit. Care 2020, 24, 129. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; Rowe, S. Factors affecting vitamin C status and prevalence of deficiency: A global health perspective. Nutrients 2020, 12, 1963. [Google Scholar] [CrossRef]
- Patterson, G.; Isales, C.M.; Fulzele, S. Low level of vitamin C and dysregulation of vitamin C transporter might be involved in the severity of COVID-19 Infection. Aging Dis. 2020, 12. [Google Scholar]
- Michels, A.J.; Joisher, N.; Hagen, T.M. Age-related decline of sodium-dependent ascorbic acid transport in isolated rat hepatocytes. Arch. Biochem. Biophys. 2003, 410, 112–120. [Google Scholar] [CrossRef]
- Subramanian, V.S.; Sabui, S.; Subramenium, G.A.; Marchant, J.S.; Said, H.M. Tumor Necrosis Factor alpha (TNF-alpha) reduces intestinal vitamin C uptake: A role for NF-kB-mediated signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G241–G248. [Google Scholar] [CrossRef]
- Subramanian, V.S.; Sabui, S.; Moradi, H.; Marchant, J.S.; Said, H.M. Inhibition of intestinal ascorbic acid uptake by lipopolysaccharide is mediated via transcriptional mechanisms. Biochim. Biophys. Acta Biomembr. 2018, 1860, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Domain-Specific Appendix: VITAMIN, C. REMAP-CAP: Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia 2020. Available online: https://static1.squarespace.com/static/5cde3c7d9a69340001d79ffe/t/5f1bba732cda7f10310643fe/1595652735252/REMAP-CAP+Vitamin+C+Domain+Specific+Appendix+V2+-+08+June+2020_WM.pdf (accessed on 26 September 2020).
- Vizcaychipi, M.P.; Shovlin, C.L.; McCarthy, A.; Howard, A.; Brown, A.; Hayes, M.; Singh, S.; Christie, L.; Sisson, A.; Davies, R.; et al. Development and implementation of a COVID-19 near real-time traffic light system in an acute hospital setting. Emerg. Med. J. 2020, 37, 630–636. [Google Scholar] [CrossRef] [PubMed]
- ICNARC Report on COVID-19 in Critical Care: Chelsea and Westminster Hospital Intensive Care Unit. London. 2020. Available online: https://www.patrickholford.com/uploads/2020/chelwesticnarcreportjune.pdf (accessed on 12 June 2020).
- ICNARC Report on COVID-19 in Critical Care. London. 2020. Available online: https://www.patrickholford.com/uploads/2020/nationwideicnarcreportjune.pdf (accessed on 26 June 2020).
- Mosdol, A.; Erens, B.; Brunner, E.J. Estimated prevalence and predictors of vitamin C deficiency within UK’s low-income population. J. Public Health 2008, 30, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Hiedra, R.; Lo, K.B.; Elbashabsheh, M.; Gul, F.; Wright, R.M.; Albano, J.; Azmaiparashvili, Z.; Patarroyo Aponte, G. The use of IV vitamin C for patients with COVID-19: A case series. Expert Rev. Anti Infect. Ther. 2020, 18, 1259–1261. [Google Scholar] [CrossRef] [PubMed]
- Waqas Khan, H.M.; Parikh, N.; Megala, S.M.; Predeteanu, G.S. Unusual early recovery of a critical COVID-19 patient after administration of intravenous vitamin C. Am. J. Case Rep. 2020, 21, e925521. [Google Scholar] [PubMed]
- Vitamin C: Fact Sheet for Health Professionals USA: National Institutes of Health. 2020. Available online: https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/ (accessed on 10 October 2020).
- Scientific Committee on Food Scientific Panel on Dietetic Products Nutrition and Allergies. Tolerable Upper Intake Levels for Vitamins and Minerals; EFSA: Parma, Italy, 2006. [Google Scholar]
- Phoenix Labs. Ascorbic Acid Injection 500mg/5ml Clonee, Ireland 2014. Available online: https://www.medicines.org.uk/emc/product/1520/smpc#gref (accessed on 23 November 2020).
- Marik, P.E. Is intravenous vitamin C contraindicated in patients with G6PD deficiency? Crit. Care 2019, 23, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerster, H. High-dose vitamin C: A risk for persons with high iron stores? Int. J. Vitam. Nutr. Res. 1999, 69, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, R.F. Vitamin C, titrating to bowel tolerance, anascorbemia, and acute induced scurvy. Med. Hypotheses 1981, 7, 1359–1376. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Sun, A.Y.; Chen, Q.; Espey, M.G.; Drisko, J.; Levine, M. Vitamin C: Intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS ONE 2010, 5, e11414. [Google Scholar] [CrossRef] [Green Version]
- Auer, B.L.; Auer, D.; Rodgers, A.L. The effect of ascorbic acid ingestion on the biochemical and physicochemical risk factors associated with calcium oxalate kidney stone formation. Clin. Chem. Lab. Med. 1998, 36, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Curhan, G.C.; Willett, W.C.; Speizer, F.E.; Stampfer, M.J. Intake of vitamins B6 and C and the risk of kidney stones in women. J. Am. Soc. Nephrol. 1999, 10, 840–845. [Google Scholar]
- Jiang, K.; Tang, K.; Liu, H.; Xu, H.; Ye, Z.; Chen, Z. Ascorbic acid supplements and kidney stones incidence among men and women: A systematic review and meta-analysis. Urol. J. 2018, 16, 115–120. [Google Scholar]
- Robitaille, L.; Mamer, O.A.; Miller, W.H., Jr.; Levine, M.; Assouline, S.; Melnychuk, D.; Rousseau, C.; Hoffer, L.J. Oxalic acid excretion after intravenous ascorbic acid administration. Metabolism 2009, 58, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, e000085. [Google Scholar] [CrossRef] [PubMed]
- Jovic, T.H.; Ali, S.R.; Ibrahim, N.; Jessop, Z.M.; Tarassoli, S.P.; Dobbs, T.D.; Holford, P.; Thornton, C.A.; Whitaker, I.S. Could vitamins help in the fight against COVID-19? Nutrients 2020, 12, 2550. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef] [Green Version]

| Study Type | Cohort | Vitamin C (µmol/L) (% Deficient, % Hypovitaminosis C) | Refs. |
|---|---|---|---|
| Pneumonia | |||
| Case control | Healthy volunteers (n = 50) | 56 ± 2 a (0% b, 8% c) | [24] |
| Community-acquired pneumonia (n = 50) | 23 ± 3 (22%, 62%) | ||
| Case control | Healthy volunteers (n = 20) | 66 ± 3 | [25] |
| Pneumonia cases (n = 11) | 31 ± 9 | ||
| Case control | Healthy participants (n = 28) | 49 ± 1 | [26] |
| Lobular pneumonia (n = 35): | |||
| Acute—did not survive (n = 7) | 17 ± 1 | ||
| Acute—survived (n = 15) | 24 ± 1 | ||
| Convalescent cases (n = 13) | 34 ± 1 | ||
| Intervention (placebo group) | Pneumonia/bronchitis (n = 29): | [18] | |
| Week 0 | 24 ± 5 (40%) b | ||
| Week 2 | 19 ± 3 (37%) | ||
| Week 4 | 24 ± 6 (25%) | ||
| Intervention (control group) | Pneumonia cases (n = 70): | [27] | |
| Day 0 | 41 | ||
| Day 5–10 | 23–24 | ||
| Day 15–20 | 32–35 | ||
| Day 30 | 39 | ||
| Sepsis | |||
| Intervention (baseline) | Sepsis with ARDS (n = 83): | [28] | |
| Day 0 | 22 (11–37) d | ||
| Day 2 | 23 (9–37) | ||
| Day 4 | 26 (9–41) | ||
| Day 7 | 29 (12–39) | ||
| Observational | Septic shock patients (n = 24) | 15 ± 2 (38% b, 88% c) | [29] |
| Intervention (baseline) | Severe sepsis patients (n = 24) | 18 ± 2 | [30] |
| Case control | Healthy controls (n = 6) | 48 ± 6 | [31] |
| Severe sepsis (n = 19) | 14 ± 3 | ||
| Septic shock (n = 37) | 14 ± 3 | ||
| Case control | Healthy controls (n = 14) | 76 ± 6 | [32] |
| Septic encephalopathy (n = 11) | 19 ± 11 | ||
| Case control | Healthy controls (n = 34) | 62 (55–72) d | [33] |
| ICU (injury, surgery, sepsis) (n = 62) | 11 (8–22) | ||
| Severe COVID-19 | |||
| Observational | Critically ill COVID-19 (n = 21) | 22 ± 4 (45%b, 70% c) e | [34] |
| Survivors (n = 11) | 29 ± 7 (40%, 50%) | ||
| Non-survivors (n = 10) | 15 ± 2 (50%, 90%) | ||
| Observational | COVID-associated ARDS (n = 18) | 17 with <9 µmol/L | [35] |
| 1 with 14 µmol/L |
| Patients | Intervention Dose (Duration) | Patient Outcomes | Refs. |
|---|---|---|---|
| Pneumonia | |||
| Pneumonia/bronchitis (n = 57): | Oral vitamin C (28 day): | ↓ respiratory symptom score in most severely ill | [18] |
| • Placebo (n = 29) | 0 g/day | 17% mortality in placebo group | |
| • Treatment (n = 28) | 0.2 g/day | 4% mortality in treatment group | |
| Pneumonia (n = 140): | Oral vitamin C (10 day): | ↓ hospital length of stay: | [27] |
| • Control (n = 70) | 0 g/day | 24 days in control group | |
| • Low dose (n = 39) | 0.25–0.8 g/day | 19 days in low dose group | |
| • High dose (n = 31) | 0.5–1.6 g/day | 15 days in high dose group | |
| Sepsis | |||
| Sepsis and ARDS (n = 167): | IV vitamin C (4 day): | X systemic organ failure score X C-reactive protein, thrombomodulin X ventilator-free days ↓ 28 day mortality ↑ ICU-free days ↑ hospital-free days | [28] |
| • Placebo (n = 83) | 0 mg/kg bw/day | ||
| • Treatment (n = 84) | 200 mg/kg/day | ||
| Septic shock (n = 100): | IV vitamin C (until ICU discharge) | ↓ vasopressor duration ↓ ICU length of stay X length of mechanical ventilation X renal replacement therapy X ICU mortality | [80] |
| • Placebo (n = 50) | 0 g/day | ||
| • Treatment (n = 50) | 6 g/day | ||
| Septic shock (n = 28): | IV vitamin C (3 day): | ↓ norepinephrine dose and duration ↓ 28 day mortality X ICU length of stay | [81] |
| • Placebo (n = 14) | 0 mg/kg bw/day | ||
| • Treatment (n = 14) | 100 mg/kg bw/day | ||
| Severe sepsis (n = 24) | IV vitamin C (4 day): | ↓ systemic organ failure score ↓ C-reactive protein, procalcitonin, thrombomodulin | [30] |
| • Placebo (n = 8) | 0 mg/kg bw/day | ||
| • Low dose (n = 8) | 50 mg/kg bw/day | ||
| • High dose (n = 8) | 200 mg/kg bw/day | ||
| Severe COVID-19 | |||
| Critical COVID-19 (n = 54) | IV vitamin C (7 day): | X ventilation-free days ↑ PaO2/FiO2 ↓ Interleukin-6 ↓ 28 day mortality in patients with SOFA scores ≥ 3 | [82] |
| • Placebo (n = 28) | 0 g/day | ||
| • Treatment (n = 26) | 24 g/day | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holford, P.; Carr, A.C.; Jovic, T.H.; Ali, S.R.; Whitaker, I.S.; Marik, P.E.; Smith, A.D. Vitamin C—An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients 2020, 12, 3760. https://doi.org/10.3390/nu12123760
Holford P, Carr AC, Jovic TH, Ali SR, Whitaker IS, Marik PE, Smith AD. Vitamin C—An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients. 2020; 12(12):3760. https://doi.org/10.3390/nu12123760
Chicago/Turabian StyleHolford, Patrick, Anitra C. Carr, Thomas H. Jovic, Stephen R. Ali, Iain S. Whitaker, Paul E. Marik, and A. David Smith. 2020. "Vitamin C—An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19" Nutrients 12, no. 12: 3760. https://doi.org/10.3390/nu12123760
APA StyleHolford, P., Carr, A. C., Jovic, T. H., Ali, S. R., Whitaker, I. S., Marik, P. E., & Smith, A. D. (2020). Vitamin C—An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients, 12(12), 3760. https://doi.org/10.3390/nu12123760






