Does Evidence Exist to Blunt Inflammatory Response by Nutraceutical Supplementation during COVID-19 Pandemic? An Overview of Systematic Reviews of Vitamin D, Vitamin C, Melatonin, and Zinc
Abstract
:1. Introduction
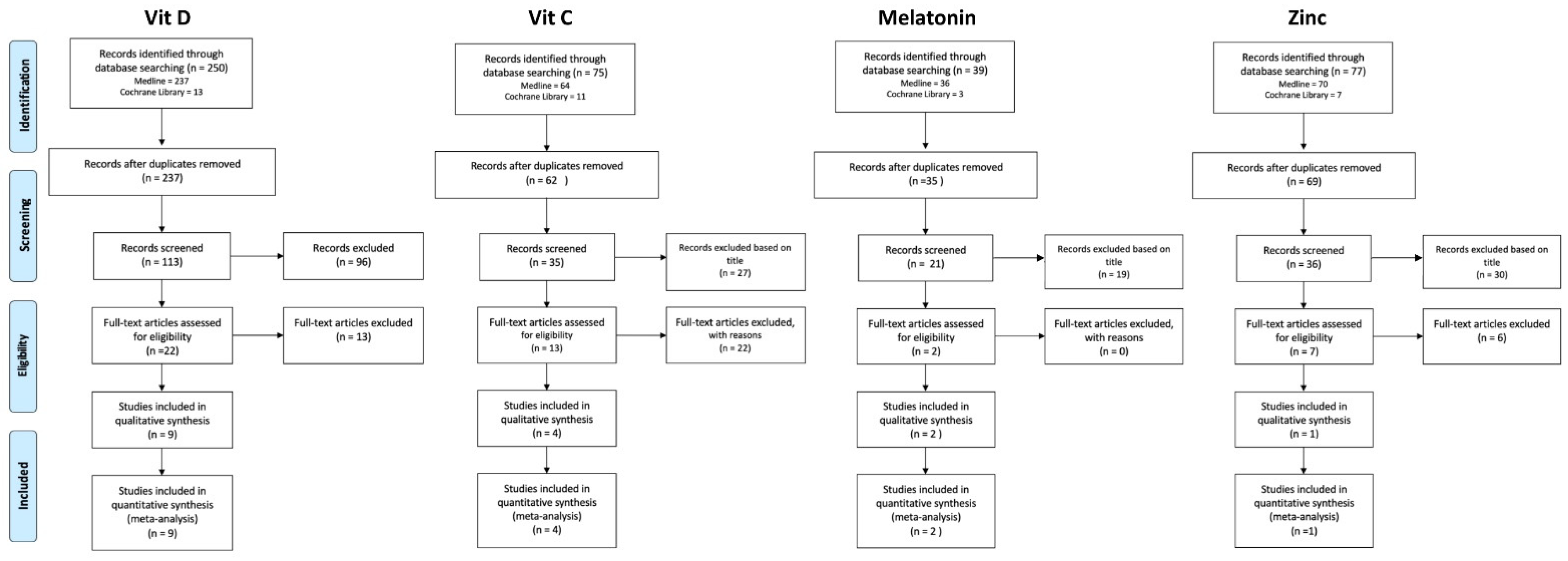
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Methods
2.2.1. Melatonin
2.2.2. Vitamin C
2.2.3. Vitamin D
2.2.4. Zinc
2.3. Study Selection
2.4. Data Extraction, Coding and Analysis
2.5. Quality Assessment of Included Reviews
2.6. Dosage of Nutraceuticals
3. Results
4. Discussion
4.1. Vitamin D
4.2. Vitamin C
4.3. Melatonin
4.4. Zinc
4.5. Evidence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watkins, J. Preventing a covid-19 pandemic. BMJ 2020, 368, m810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Hussain, A.; Misra, A. Diabetes and COVID-19: Evidence, current status and unanswered research questions. Eur. J. Clin. Nutr. 2020, 74, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. J. Am. Med. Assoc. 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Who Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 23 January 2021).
- Li, Q.; Guan, X.; Wu, P.; Qun, L.; Xuhua, G.; Peng, W.; Xiaoye, W.; Lei, Z.; Yeqing, T.; Ruiqi, R.; et al. Early transmission dynamics in Wuhan, China, of Novel Coronavirus—Infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125 (Suppl. S2), S3–S23. [Google Scholar] [CrossRef]
- Zabetakis, I.; Lordan, R.; Norton, C.; Tsoupras, A. COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients 2020, 12, 1466. [Google Scholar] [CrossRef]
- Maggini, S.; Beveridge, S.; Sorbara, P.J.P.; Senatore, G. Feeding the immune system: The role of micronutrients in restoring resistance to infections. CAB Rev. 2008, 3, 1–21. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Herold, T.; Jurinovic, V.; Arnreich, C.; Lipworth, B.J.; Hellmuth, J.C.; Von Bergwelt-Baildon, M.; Klein, M.; Weinberger, T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020, 146, 128–136.e4. [Google Scholar] [CrossRef]
- Asbaghi, O.; Sadeghian, M.; Mozaffari-Khosravi, H.; Maleki, V.; Shokri, A.; Hajizadeh-Sharafabad, F.; Alizadeh, M.; Sadeghi, O. The effect of vitamin d-calcium co-supplementation on inflammatory biomarkers: A systematic review and meta-analysis of randomized controlled trials. Cytokine 2020, 129, 155050. [Google Scholar] [CrossRef]
- Fisher, S.A.; Rahimzadeh, M.; Brierley, C.; Gration, B.; Doree, C.; Kimber, C.E.; Cajide, A.P.; Lamikanra, A.A.; Roberts, D.J. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: A systematic review. PLoS ONE 2019, 14, e0222313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Liu, M.; Wang, C.; Xiao, Y.; An, T.; Zou, M.; Cheng, G. Association between vitamin D status and asthma control: A meta-analysis of randomized trials. Respir. Med. 2019, 150, 85–94. [Google Scholar] [CrossRef]
- Yu, Y.; Tian, L.; Xiao, Y.; Huang, G.; Zhang, M. Effect of Vitamin D Supplementation on Some Inflammatory Biomarkers in Type 2 Diabetes Mellitus Subjects: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Nutr. Metab. 2018, 73, 62–73. [Google Scholar] [CrossRef]
- Mazidi, M.; Rezaie, P.; Vatanparast, H. Impact of vitamin D supplementation on C-reactive protein; a systematic review and meta-analysis of randomized controlled trials. BMC Nutr. 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calton, E.K.; Keane, K.N.; Newsholme, P.; Zhao, Y.; Soares, M.J. The impact of cholecalciferol supplementation on the systemic inflammatory profile: A systematic review and meta-analysis of high-quality randomized controlled trials. Eur. J. Clin. Nutr. 2017, 71, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Tari, A.; Freeborn, E.; Mushtaq, E. Impact of vitamin D supplementation on endothelial and inflammatory markers in adults: A systematic review. J. Steroid. Biochem. Mol. Biol. 2017, 173, 292–300. [Google Scholar]
- Jamka, M.; Woźniewicz, M.; Walkowiak, J.; Bogdański, P.; Jeszka, J.; Stelmach-Mardas, M. The effect of vitamin D supplementation on selected inflammatory biomarkers in obese and overweight subjects: A systematic review with meta-analysis. Eur. J. Nutr. 2016, 55, 2163–2176. [Google Scholar] [CrossRef]
- Chen, N.; Wan, Z.; Han, S.; Li, B.; Zhang, Z.; Qin, L. Effect of Vitamin D Supplementation on the Level of Circulating High-Sensitivity C-Reactive Protein: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2014, 6, 2206–2216. [Google Scholar] [CrossRef] [Green Version]
- Safabakhsh, M.; Emami, M.R.; Khosroshahi, M.Z.; Asbaghi, O.; Khodayari, S.; Khorshidi, M.; Alizadeh, S.; Viri, E.H. Vitamin C supplementation and C-reactive protein levels: Findings from a systematic review and meta-analysis of clinical trials. J. Complement. Integr. Med. 2020. [Google Scholar] [CrossRef]
- Jafarnejad, S.; Boccardi, V.; Hosseini, B.; Taghizadeh, M.; Hamedifard, Z. A Meta-analysis of Randomized Control Trials: The Impact of Vitamin C Supplementation on Serum CRP and Serum hs-CRP Concentrations. Curr. Pharm. Des. 2018, 24, 3520–3528. [Google Scholar] [CrossRef]
- Ashor, A.W.; Siervo, M.; Lara, J.; Oggioni, C.; Afshar, S.; Mathers, J.C. Effect of vitamin C and vitamin E supplementation on endothelial function: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 113, 1182–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashor, A.W.; Lara, J.; Mathers, J.C.; Siervo, M. Effect of vitamin C on endothelial function in health and disease: A systematic review and meta-analysis of randomised controlled trials. Atherosclerosis 2014, 235, 9–20. [Google Scholar] [CrossRef]
- Biniaz, V.; Shermeh, M.S.; Ebadi, A.; Tayebi, A.; Einollahi, B. Effect of Vitamin C Supplementation on C-reactive Protein Levels in Patients Undergoing Hemodialysis: A Randomized, Double Blind, Placebo-Controlled Study. Nephro-Urol. Mon. 2013, 6, e13351. [Google Scholar] [CrossRef] [Green Version]
- Attallah, N.; Osman-Malik, Y.; Frinak, S.; Besarab, A. Effect of Intravenous Ascorbic Acid in Hemodialysis Patients with EPO-Hyporesponsive Anemia and Hyperferritinemia. Am. J. Kidney Dis. 2006, 47, 644–654. [Google Scholar] [CrossRef]
- De Marchi, S.; Prior, M.; Rigoni, A.; Zecchetto, S.; Rulfo, F.; Arosio, E. Ascorbic acid prevents vascular dysfunction induced by oral glucose load in healthy subjects. Eur. J. Intern. Med. 2012, 23, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Colby, J.A.; Chen, W.T.; Baker, W.L.; Coleman, C.I.; Reinhart, K.; Kluger, J.; White, C.M. Effect of ascorbic acid on inflammatory markers after cardiothoracic surgery. Am. J. Heal. Pharm. 2011, 68, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Modi, J.; Modi, P.; Pal, B.; Nagarajan, R.; Saifee, Y.; Bansal, J.; Kumar, S. Role of Vitamin C and E supplementation in reduction of serum level of renal injury marker following shock wave lithotripsy: Prospective single centre experience. Urol. Ann. 2015, 7, 350–354. [Google Scholar] [CrossRef]
- Antoniades, C.; Tousoulis, D.; Tountas, C.; Tentolouris, C.; Toutouza, M.; Vasiliadou, C.; Tsioufis, C.; Toutouzas, P.; Stefanadis, C. Vascular endothelium and inflammatory process, in patients with combined Type 2 diabetes mellitus and coronary atherosclerosis: The effects of vitamin C. Diabet. Med. 2004, 21, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Antoniades, C.; Vasiliadou, C.; Kourtellaris, P.; Koniari, K.; Marinou, K.; Charakida, M.; Ntarladimas, I.; Siasos, G.; Stefanadis, C. Effects of atorvastatin and vitamin C on forearm hyperaemic blood flow, asymmentrical dimethylarginine levels and the inflammatory process in patients with type 2 diabetes mellitus. Heart 2007, 93, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Zarezadeh, M.; Khorshidi, M.; Emami, M.; Janmohammadi, P.; Kord-Varkaneh, H.; Mousavi, S.M.; Mohammed, S.H.; Saedisomeolia, A.; Alizadeh, S. Melatonin supplementation and pro-inflammatory mediators: A systematic review and meta-analysis of clinical trials. Eur. J. Nutr. 2020, 59, 1803–1813. [Google Scholar] [CrossRef]
- Akbari, M.; Ostadmohammadi, V.; Tabrizi, R.; Lankarani, K.B.; Heydari, S.T.; Amirani, E.; Reiter, R.J.; Asemi, Z. The effects of melatonin supplementation on inflammatory markers among patients with metabolic syndrome or related disorders: A systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology 2018, 26, 899–907. [Google Scholar] [CrossRef]
- Sánchez-López, A.; Ortiz, G.G.; Pacheco-Moises, F.P.; Mireles-Ramírez, M.A.; Bitzer-Quintero, O.K.; Delgado-Lara, D.L.C.; Ramírez-Jirano, L.J.; Velázquez-Brizuela, I.E. Efficacy of Melatonin on Serum Pro-inflammatory Cytokines and Oxidative Stress Markers in Relapsing Remitting Multiple Sclerosis. Arch. Med. Res. 2018, 49, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Paolorossi, F.; Tancini, G.; Barni, S.; Ardizzoia, A.; Brivio, F.; Zubelewicz, B.; Chatikhine, V. Is there a role for melatonin in the treatment of neoplastic cachexia? Eur. J. Cancer 1996, 32, 1340–1343. [Google Scholar] [CrossRef]
- Raygan, F.; Ostadmohammadi, V.; Bahmani, F.; Reiter, R.J.; Asemi, Z. Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Pakravan, H.; Ahmadian, M.; Fani, A.; Aghaee, D.; Brumanad, S.; Pakzad, B. The Effects of melatonin in patients with nonalcoholic fatty liver disease: A randomized controlled trial. Adv. Biomed. Res. 2017, 6, 40. [Google Scholar]
- Javanmard, S.; Heshmat-Ghahdarijani, K.; Mirmohammad-Sadeghi, M.; Sonbolestan, S.A.; Ziayi, A. The effect of melatonin on endothelial dysfunction in patient undergoing coronary artery bypass grafting surgery. Adv. Biomed. Res. 2016, 5, 174. [Google Scholar]
- Forrest, C.M.; Mackay, G.M.; Stoy, N.; Stone, T.W.; Darlington, L.G. Inflammatory status and kynurenine metabolism in rheumatoid arthritis treated with melatonin. Br. J. Clin. Pharmacol. 2007, 64, 517–526. [Google Scholar] [CrossRef] [Green Version]
- Celinski, K.; Konturek, P.; Slomka, M.; Cichoz-Lach, H.; Brzozowski, T.; Konturek, S.J.; Korolczuk, A. Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with nonalcoholic fatty liver disease–14 months follow up. J. Physiol. Pharmacol. 2014, 65, 75–82. [Google Scholar]
- Cichoz-Lach, H.; Celinski, K.; Konturek, P.C.; Konturek, S.J.; Slomka, M. The effects of l-tryptophan and melatonin on selected biochemical parameters in patients with steatohepatitis. J. Physiol. Pharmacol. 2010, 61, 577–580. [Google Scholar]
- Alamdari, N.M.; Mahdavi, R.; Roshanravan, N.; Yaghin, N.L.; Ostadrahimi, A.R.; Faramarzi, E. A Double-Blind, Placebo-Controlled Trial Related to the Effects of Melatonin on Oxidative Stress and Inflammatory Parameters of Obese Women. Horm. Metab. Res. 2015, 47, 504–508. [Google Scholar] [CrossRef] [Green Version]
- Chojnacki, C.; Wisniewska-Jarosinska, M.; Walecka-Kapica, E.; Klupinska, G.; Jaworek, J.; Chojnacki, J. Evaluation of melatonin effectiveness in the adjuvant treatment of ulcerative colitis. J. Physiol. Pharmacol. 2011, 62, 327–334. [Google Scholar]
- Mousavi, S.M.; Djafarian, K.; Mojtahed, A.; Varkaneh, H.K.; Shab-Bidar, S. The effect of zinc supplementation on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Pharmacol. 2018, 834, 10–16. [Google Scholar] [CrossRef]
- Rashidi, A.A.; Salehi, M.; Piroozmand, A.; Sagheb, M.M. Effects of zinc supplementation on serum zinc and C-reactive protein concentrations in hemodialysis patients. J. Ren. Nutr. 2009, 19, 475–478. [Google Scholar] [CrossRef]
- Pourteymour Fard Tabrizi, F.; Alipoor, B.; Ostadrahimi, A.R.; Mehrzad Sadagiani, M. Effect of zinc supplementation on inflammatory markers in women with polycystic ovary syndrome. Shiraz E Med. J. 2011, 12, 30–38. [Google Scholar]
- Jamilian, M.; Foroozanfard, F.; Bahmani, F.; Talaee, R.; Monavari, M.; Asemi, Z. Effects of Zinc Supplementation on Endocrine Outcomes in Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2016, 170, 271–278. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [Green Version]
- Cavezzi, A.; Troiani, E.; Corrao, S. COVID-19: Hemoglobin, Iron, and Hypoxia beyond Inflammation. A Narrative Review. Clin. Pr. 2020, 10, 24–30. [Google Scholar] [CrossRef]
- Bandeira, F.; Griz, L.; Dreyer, P.; Eufrazino, C.; Bandeira, C.; Freese, E. Vitamin D deficiency: A global perspective. Arq. Bras. Endocrinol. Metabol. 2006, 50, 640–646. [Google Scholar] [CrossRef] [Green Version]
- Shahid, Z.; Bs, R.K.; Bs, B.M.; Kepko, D.; Bs, D.R.; Patel, R.; Mbbs, C.S.A.; Vunnam, R.R.; Sahu, N.; Bhatt, D.; et al. COVID-19 and Older Adults: What We Know. J. Am. Geriatr. Soc. 2020, 68, 926–929. [Google Scholar] [CrossRef] [Green Version]
- Ragab, D.; Eldin, H.S.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, M.; Hung, M. Men and COVID-19: A pathophysiologic review. Am. J. Mens Health 2020, 14, 1557988320954021. [Google Scholar] [CrossRef]
- Cutolo, M.; Paolino, S.; Smith, V. Evidences for a protective role of vitamin D in COVID-19. RMD Open 2020, 6, e001454. [Google Scholar] [CrossRef] [PubMed]
- Cyprian, F.; Lefkou, E.; Varoudi, K.; Girardi, G. Immunomodulatory Effects of Vitamin D in Pregnancy and Beyond. Front. Immunol. 2019, 10, 2739. [Google Scholar] [CrossRef] [Green Version]
- Rondanelli, M.; Miccono, A.; Lamburghini, S.; Avanzato, I.; Riva, A.; Allegrini, P.; Faliva, M.A.; Peroni, G.; Nichetti, M.; Perna, S. Self-Care for Common Colds: The Pivotal Role of Vitamin D, Vitamin C, Zinc, and Echinacea in Three Main Immune Interactive Clusters (Physical Barriers, Innate and Adaptive Immunity) Involved during an Episode of Common Colds—Practical Advice on Dosages and on the Time to Take These Nutrients/Botanicals in order to Prevent or Treat Common Colds. Evid. Based Complement. Altern. Med. 2018, 2018, 5813095. [Google Scholar]
- Schwalfenberg, G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol. Nutr. Food Res. 2011, 55, 96–108. [Google Scholar] [CrossRef]
- Kast, J.I.; McFarlane, A.J.; Głobin’ska, A.; Sokolowska, M.; Wawrzyniak, P.; Sanak, M.; Schwarze, J.; Akdis, C.A.; Wanke, K. Respiratory syncytial virus infection influences tight junction integrity. Clin. Exp. Immunol. 2017, 190, 351–359. [Google Scholar] [CrossRef] [Green Version]
- McCartney, D.M.; Byrne, D.G. Optimisation of vitamin D status for enhanced immuno-protection against CoViD-19. Ir. Med. J. 2020, 113, 58. [Google Scholar]
- Vankadari, N.; Wilce, J.A. Emerging WuHan (COVID-19) coronavirus: Glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microbes Infect. 2020, 9, 601–604. [Google Scholar] [CrossRef]
- Skariyachan, S.; Challapilli, S.B.; Packirisamy, S.; Kumargowda, S.T.; Sridhar, V.S. Recent aspects on the pathogenesis mechanism, animal models and novel therapeutic interventions for middle-east respiratory syndrome coronavirus infections. Front. Microbiol. 2019, 10, 569. [Google Scholar] [CrossRef] [Green Version]
- Beard, J.A.; Bearden, A.; Striker, R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011, 50, 194–200. [Google Scholar] [CrossRef]
- Teymoori-Rad, M.; Shokri, F.; Salimi, V.; Marashi, S.M. The interplay between vitamin D and viral infections. Rev. Med Virol. 2019, 29, e2032. [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Der Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and Multiorgan Response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar] [CrossRef]
- Gagnon, C.; Daly, R.M.; Carpentier, A.; Lu, Z.X.; Shore-Lorenti, C.; Sikaris, K.; Jean, S.; Ebeling, P.R. Effects of Combined Calcium and Vitamin D Supplementation on Insulin Secretion, Insulin Sensitivity and β-Cell Function in Multi-Ethnic Vitamin D-Deficient Adults at Risk for Type 2 Diabetes: A Pilot Randomized, Placebo-Controlled Trial. PLoS ONE 2014, 9, e109607. [Google Scholar] [CrossRef] [PubMed]
- Aihara, K.-I.; Azuma, H.; Akaike, M.; Ikeda, Y.; Yamashita, M.; Sudo, T.; Hayashi, H.; Yamada, Y.; Endoh, F.; Fujimura, M.; et al. Disruption of Nuclear Vitamin D Receptor Gene Causes Enhanced Thrombogenicity in Mice. J. Biol. Chem. 2004, 279, 35798–35802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu-Wong, J.R.; Nakane, M.; Ma, J. Vitamin D Analogs Modulate the Expression of Plasminogen Activator Inhibitor-1, Thrombospondin-1 and Thrombomodulin in Human Aortic Smooth Muscle Cells. J. Vasc. Res. 2007, 44, 11–18. [Google Scholar] [CrossRef]
- Gomes, T.; Várady, C.B.S.; Lourenço, A.L.; Mizurini, D.M.; Rondon, A.M.R.; Leal, A.C.; Gonçalves, B.S.; Bou-Habib, D.C.; Medei, E.; Monteiro, R.Q. IL-1β Blockade Attenuates Thrombosis in a Neutrophil Extracellular Trap-Dependent Breast Cancer Model. Front. Immunol. 2019, 10, 2088. [Google Scholar] [CrossRef] [Green Version]
- Erdei, J.; Tóth, A.; Balogh, E.; Nyakundi, B.B.; Bányai, E.; Ryffel, B.; Paragh, G.; Cordero, M.D.; Jeney, V. Induction of NLRP3 Inflammasome Activation by Heme in Human Endothelial Cells. Oxidative Med. Cell. Longev. 2018, 2018, 4310816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, G.-S.; Zhang, C.; Cheng, B.; Lee, C.-H. Mechanisms of Action of Vitamin D as Supplemental Therapy for Pneumocystis Pneumonia. Antimicrob. Agents Chemother. 2017, 61, e01226-17. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Immunomodulatory and Antimicrobial Effects of Vitamin C. Eur. J. Microbiol. Immunol. 2019, 9, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.; Berrill, M.; Marik, P. The antiviral properties of vitamin C. Expert Rev. Anti-Infect. Ther. 2019, 18, 99–101. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Lin, Y.D.; Yang, L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.K.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D 3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and foxp3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Wu, H.; Wang, C.; Xiao, Z.; Xu, F. Regulatory T cells and acute lung injury: Cytokines, uncontrolled inflammation, and therapeutic implications. Front. Immunol. 2018, 9, 1545. [Google Scholar] [CrossRef] [Green Version]
- Giannini, S.; Passeri, G.; Tripepi, G.; Sella, S.; Fusaro, M.; Arcidiacono, G.; Torres, M.O.; Michielin, A.; Prandini, T.; Baffa, V.; et al. Effectiveness of In-Hospital Cholecalciferol Use on Clinical Outcomes in Comorbid COVID-19 Patients: A Hypothesis-Generating Study. Nutrients 2021, 13, 219. [Google Scholar] [CrossRef]
- Castillo, M.E.; Costa, L.M.E.; Barrios, J.M.V.; Díaz, J.F.A.; Miranda, J.L.; Bouillon, R.; Gomez, J.M.Q. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid. Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Teshome, A.; Adane, A.; Girma, B.; Mekonnen, Z.A. The Impact of Vitamin D Level on COVID-19 Infection: Systematic Review and Meta-Analysis. Front. Public Heal. 2021, 9, 624559. [Google Scholar] [CrossRef]
- Pereira, M.; Dantas Damascena, A.; Galvão Azevedo, L.M.; de Almeida Oliveira, T.; da Mota Santana, J. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 1–9. [Google Scholar] [CrossRef]
- Hemilä, H. Vitamin C and SARS coronavirus. J. Antimicrob. Chemother. 2003, 52, 1049–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, J.-Y.; Kim, S.-K. Quercetin and Ascorbic Acid Suppress Fructose-Induced NLRP3 Inflammasome Activation by Blocking Intracellular Shuttling of TXNIP in Human Macrophage Cell Lines. Inflammation 2017, 40, 980–994. [Google Scholar] [CrossRef] [PubMed]
- Hemila, H. Vitamin C supplementation and respiratory infections: A systematic review. Mil. Med. 2004, 169, 920–925. [Google Scholar] [CrossRef] [Green Version]
- Boretti, A.; Bimal, K. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition 2020, 12, 100190. [Google Scholar] [CrossRef]
- Jovic, T.H.; Ali, S.R.; Ibrahim, N.; Jessop, Z.M.; Tarassoli, S.P.; Dobbs, T.D.; Holford, P.; Thornton, C.A.; Whitaker, I.S. Could Vitamins Help in the Fight Against COVID-19? Nutrients 2020, 12, 2550. [Google Scholar] [CrossRef]
- Saleh, J.; Peyssonnaux, C.; Singh, K.K.; Edeas, M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion 2020, 54, 1–7. [Google Scholar] [CrossRef]
- Cantoni, O.; Guidarelli, A.; Fiorani, M. Mitochondrial Uptake and Accumulation of Vitamin C: What Can We Learn from Cell Culture Studies? Antioxidants Redox Signal. 2018, 29, 1502–1515. [Google Scholar] [CrossRef] [PubMed]
- Kc, S.; Càrcamo, J.M.; Golde, D.W. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Gluti) and confers mitochondrial protection against oxidative injury. FASEB J. 2005, 19, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- VanDuijn, M.M.; Tijssen, K.; VanSteveninck, J.; Broek, P.J.V.D.; Van der Zee, J. Erythrocytes Reduce Extracellular Ascorbate Free Radicals Using Intracellular Ascorbate as an Electron Donor. J. Biol. Chem. 2000, 275, 27720–27725. [Google Scholar] [CrossRef] [Green Version]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C Pharmacokinetics: Implications for Oral and Intravenous Use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef]
- Fonorow, O.; Hickey, S. Unexpected Early Response in Oral Bioavailability of Ascorbic Acid. Townsend Lett. 2020, 52. Available online: https://www.townsendletter.com/article/online (accessed on 13 March 2021).
- Richard, Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med. Drug Discov. 2020, 5, 100028. [Google Scholar]
- Cerullo, G.; Negro, M.; Parimbelli, M.; Pecoraro, M.; Perna, S.; Liguori, G.; Rondanelli, M.; Cena, H.; D’Antona, G. The Long History of Vitamin C: From Prevention of the Common Cold to Potential Aid in the Treatment of COVID-19. Front. Immunol. 2020, 11, 574029. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Lu, H.; Shanghai Clinical Treatment Expert Group for Coronavirus Disease 2019. Comprehensive treatment and management of corona virus disease 2019: Expert consensus statement from Shanghai. Chin. J. Infect. Dis. 2020, 38. [Google Scholar] [CrossRef]
- Holford, P.; Carr, A.C.; Jovic, T.H.; Ali, S.R.; Whitaker, I.S.; Marik, P.E.; Smith, A.D. Vitamin C—An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients 2020, 12, 3760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; De Backer, D.; Xiang, H.; et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann. Intensiv. Care 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Dembra, S.; Dembra, P.; Bhawna, F.; Gul, A.; Ali, B.; Sohail, H.; Kumar, B.; Memon, M.K.; Rizwan, A. The Role of Vitamin C as Adjuvant Therapy in COVID-19. Cureus 2020, 12. [Google Scholar] [CrossRef]
- Zhao, B.; Ling, Y.; Li, J.; Peng, Y.; Huang, J.; Wang, Y.; Qu, H.; Gao, Y.; Li, Y.; Hu, B.; et al. Beneficial aspects of high dose intravenous vitamin C on patients with COVID-19 pneumonia in severe condition: A retrospective case series study. Ann. Palliat. Med. 2021, 10, 1599–1609. [Google Scholar] [CrossRef]
- Arslan, B.; Ergun, N.U.; Topuz, S.; Semerci, S.Y.; Suner, N.; Kocatas, A.; Onal, H. Synergistic Effect of Quercetin and Vitamin C Against COVID-19: Is a Possible Guard for Front Liners. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- ClinicalTrial.gov. U.S National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT04664010?term=vitamin+c&cond=covid-19&draw=2&rank=1 (accessed on 3 April 2021).
- ClinicalTrial.gov. U.S National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT04323514?term=vitamin+c&cond=covid-19&draw=2 (accessed on 3 April 2021).
- Scholtens, R.M.; Van Munster, B.C.; Van Kempen, M.F.; De Rooij, S.E.J.A. Physiological melatonin levels in healthy older people: A systematic review. J. Psychosom. Res. 2016, 86, 20–27. [Google Scholar] [CrossRef]
- Cavezzi, A.; Ambrosini, L.; Colucci, R.; Ionna, G.D.; Urso, S.U. Aging in the Perspective of Integrative Medicine, Psychoneuroendocrineimmunology and Hormesis. Curr. Aging Sci. 2020, 13, 82–91. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R. Potential utility of melatonin in deadly infectious diseases related to the overreaction of innate immune response and destructive inflammation: Focus on COVID-19. Melatonin Res. 2020, 3, 120–143. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Ni, L.; Di, X.; Ma, B.; Niu, S.; Liu, C.; Reiter, R.J. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020, 250, 117583. [Google Scholar] [CrossRef]
- Loh, D. The potential of melatonin in the prevention and attenuation of oxidative hemolysis and myocardial injury from cd147 SARS-CoV-2 spike protein receptor binding. Melatonin Res. 2020, 3, 380–416. [Google Scholar] [CrossRef]
- Juybari, K.B.; Pourhanifeh, M.H.; Hosseinzadeh, A.; Hemati, K.; Mehrzadi, S. Melatonin potentials against viral infections including COVID-19: Current evidence and new findings. Virus Res. 2020, 287, 198108. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Carretero, M.; Doerrier, C.; López, L.C.; García-Corzo, L.; Tresguerres, J.A.; Escames, G. Melatonin protects lung mitochondria from aging. AGE 2011, 34, 681–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrillo-Vico, A.; Lardone, P.J.; Álvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the Immune System. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volt, H.; García, J.A.; Doerrier, C.; Díaz-Casado, M.E.; Guerra-Librero, A.; López, L.C.; Escames, G.; Tresguerres, J.A.; Acuña-Castroviejo, D. Same molecule but different expression: Aging and sepsis trigger NLRP3 inflammasome activation, a target of melatonin. J. Pineal Res. 2016, 60, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Grailer, J.J.; Wang, N.; Wang, M.; Yao, J.; Zhong, R.; Gao, G.F.; Ward, P.A.; Tan, D.-X.; et al. Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J. Pineal Res. 2016, 60, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, X.; Liu, M.; Fan, H.; Zheng, H.; Zhang, S.; Rahman, N.; Wołczyński, S.; Kretowski, A.; Li, X. Melatonin inhibits inflammasome-associated activation of endothelium and macrophages attenuating pulmonary arterial hypertension. Cardiovasc. Res. 2020, 116, 2156–2169. [Google Scholar] [CrossRef]
- Sehirli, A.O.; Sayiner, S.; Serakinci, N. Role of melatonin in the treatment of COVID-19; as an adjuvant through cluster differentiation 147 (CD147). Mol. Biol. Rep. 2020, 47, 8229–8233. [Google Scholar] [CrossRef]
- Ramlall, V.; Zucker, J.; Tatonetti, N. Melatonin is significantly associated with survival of intubated COVID-19 patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Farnoosh, G.; Akbariqomi, M.; Badri, T.; Bagheri, M.; Izadi, M.; Saeedi-Boroujeni, A.; Rezaie, E.; Ghaleh, H.E.G.; Aghamollaei, H.; Fasihi-Ramandi, M.; et al. Efficacy of a Low Dose of Melatonin as an Adjunctive Therapy in Hospitalized Patients with COVID-19: A Randomized, Double-blind Clinical Trial. Authorea Prepr. 2020. [Google Scholar] [CrossRef]
- Essa, M.M.; Hamdan, H.; Chidambaram, S.B.; Al-Balushi, B.; Guillemin, G.J.; Ojcius, D.M.; Qoronfleh, M.W. Possible role of tryptophan and melatonin in COVID-19. Int. J. Tryptophan Res. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hou, Y.; Shen, J.; Mehra, R.; Kallianpur, A.; Culver, D.A.; Gack, M.U.; Farha, S.; Zein, J.; Comhair, S.; et al. A network medicine approach to investigation and population-based validation of disease manifestations and drug repurposing for COVID-19. PLoS Biol. 2020, 18, e3000970. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Can Melatonin Be a Potential “Silver Bullet” in Treating COVID-19 Patients? Diseases 2020, 8, 44. [Google Scholar] [CrossRef]
- Pal, A.; Squitti, R.; Picozza, M.; Pawar, A.; Rongioletti, M.; Dutta, A.K.; Sahoo, S.; Goswami, K.; Sharma, P.; Prasad, R. Zinc and COVID-19: Basis of Current Clinical Trials. Biol. Trace Elem. Res. 2020. [Google Scholar] [CrossRef]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2018, 9, 3160. [Google Scholar] [CrossRef]
- Shankar, A.H.; Prasad, A.S. Zinc and immune function: The biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998, 68 (Suppl. S2), 447S–463S. [Google Scholar] [CrossRef] [Green Version]
- Tomita, H. Zinc in taste and smell disorder. Trace Elem. Clin. Med. 1990, 15–37. [Google Scholar] [CrossRef]
- Velthuis, A.J.W.T.; Worm, S.H.E.V.D.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; Van Hemert, M.J. Zn2+ Inhibits Coronavirus and Arterivirus RNA Polymerase Activity In Vitro and Zinc Ionophores Block the Replication of These Viruses in Cell Culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.M.; Gallagher, T.; Weiss, S.R. Neurovirulent murine coronavirus JHM.SD uses cellular zinc metalloproteases for virus entry and cell-cell fusion. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.-S.; Chang, G.-G.; Juo, C.-G.; Lee, H.-J.; Yeh, S.-H.; Hsu, J.T.-A.; Chen, X. Papain-like protease 2 (PLP2) from severe acute respiratory syndrome coronavirus (SARS-CoV): Expression, purification, characterization, and inhibition. Biochemistry 2005, 44, 10349–10359. [Google Scholar] [CrossRef] [PubMed]
- Derwand, R.; Scholz, M.; Zelenko, V. COVID-19 outpatients: Early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: A retrospective case series study. Int. J. Antimicrob. Agents 2020, 56, 106214. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Patel, D.; Bittel, B.; Wolski, K.; Wang, Q.; Kumar, A.; Il’Giovine, Z.J.; Mehra, R.; McWilliams, C.; Nissen, S.E.; et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection. JAMA Netw. Open 2021, 4, e210369. [Google Scholar] [CrossRef] [PubMed]

| Study | Omid Asbaghia (2019) [11] | Sheila A. FisherID (2019) [12] | Mingming Wanga (2019) [13] | Yanting Yu (2018) [14] | Mohsen Mazidi (2018) [15] | EK Calton (2018) [16] | Tari Agbalalah (2017) [17] | Małgorzata Jamka (2015) [18] | Neng Chen (2014) [19] |
|---|---|---|---|---|---|---|---|---|---|
| Databases searched | PubMed, Scopus, ISI Web of Science and Google Scholar | Central, Medline, ENBASE, PubMed and Web of Science | PubMed, EMBASE, and Cochrane Library | PubMed and the Cochrane Library | PubMed-Medline, SCOPUS, Google Scholar and Web of Science | SCOPUS and PubMed | Cochrane, PubMed and Medline | PubMed, Scopus, the Cochrane Library and EMBASE | PubMed, Web of Science, and Cochrane library |
| Articles included | 8 | 8 | 14 | 13 | 24 | 9 | 29 | 13 | 10 |
| Type of patients analyzed | Healthy subjects and patients with colorectal adenoma, type 2 diabetes mellitus, pregnancy, pregnancy with gestational diabetes and polycystic ovary syndrome | Patients with type1 diabetes, Addison’s disease, multiple sclerosis, asthma and healthy subjects | Patients with asthma | Patients with type 2 diabetes | Patients with obesity, type 2 diabetic, HIV-infected, non-diabetic chronic kidney disease chronic fatigue syndrome, non-alcoholic fatty liver disease and healthy pregnant. | Patients ≥ 60 years, overweight and obese, prediabetes, non-alcoholic fatty liver disease, myocardial infarction, isolated systolic hypertension, postmenopausal women. | Patients with type 2 and gestational diabetes mellitus/prediabetic, cardiovascular disease, chronic kidney disease and overweight/obese participants | Obese and overweight subjects | Healthy subjects and patients with type 2 diabetes, polycystic ovary syndrome women, obese adults, coronary artery disease patients |
| Posology |
|
| 500 UI/day vit D |
| 400 IU/day to 11200 IU/day vit D | 200 IU/day to 11200 IU/day vit D | 4000 IU/weeks vit D | 1000 IU/day to 7000 IU/day vit D | 400 IU/day to 7000 IU/day vit D |
| Intervention duration range | 6 weeks–3 years | 3–12 months | 1,5–12 months | 8–52 weeks | 4 weeks–12 months | 12–52 weeks | 8–52 weeks | 4–52 weeks | 9–48 weeks |
| Endpoint | The effect of vitamin D–calcium co-supplementation on inflammatory biomarkers in adults | The effect of vitamin D supplementation in enhancing absolute T regulatory cells (Treg) numbers in patients with inflammatory or autoimmune disease. | To assess the correlations of vitamin D status with asthma- related respiratory outcomes. | To examine whether or not the supplementation of vitamin D exhibits anti-inflammatory benefits in T2DM subjects | To evaluate the effect of vitamin D supplementation on C-reactive protein (CRP) | Causal links between vitamin D status [25(OH)D] and systemic inflammation | The effects of vitamin D supplementation on endothelial function and inflammation in adults | The effect of supplementation with vitamin D on selected inflammatory biomarkers in overweight and obese subjects. | To evaluate the association of vitamin D supplementation with circulating hs-CRP levels. |
| Result | A significant reducing effect of vitamin D–calcium co-supplementation on serum CRP concentrations in comparison with placebo. No significant effect of joint calcium and supplementation with vitamin D on serum concentrations of IL-6 (WMD: −1.45, 95% CI: −5.31, 2.41 pg/mL, p = 0.46) and TNF-α (WMD: −0.79, 95% CI: −2.19, 0.61 pg/mL, p = 0.26). | Planned meta-analysis was not possible due to the heterogeneous nature of the studies. Nevertheless, in a trial of autoimmune disorders which measured the proportion of Tregs, a significant difference was reported, with a higher percentage of Tregs observed in the vitamin D group (at 12 weeks, mean 6.4% (SD 0.8%) (vitamin D) vs. 5.5% (1.0%) (placebo). | Vitamin D supplementation was associated with a protective effect of exacerbation in patients with vitamin D insufficiency (vitamin D < 30 ng/mL) (RR: 0.76 95%Cl (0.61–0.95)). It was also demonstrated an improvement of their FEV1% (FEV1% < 80%) (MD: 8.3 95%Cl (5.95–10.64). | Vitamin D supplementation significantly decreased the circulating hs-CRP concentration (standard mean differences, −0.45 [95% CI −0.77 to −0.14], p = 0.005). No significant effect of vitamin D supplementation on IL-6 and TNF-α plasma concentration. | The results indicated that the vitamin D supplementation significant decreased the hs-CRP level by 0.45 μg/mL, whereas the vitamin D supplementation did not influence the TNF-α and IL-6. | There was no effect on the weighted mean difference (WMD) of IL-6 [(WMD (95% confidence interval) = 0.1, (−0.166, 0.366) pg/mL, p = 0.462)] or C-reactive protein (CRP) [(WMD = −0.324, (−1.007, 0.359) mg/L, p = 0.352)]. | No significant change in both endothelial and inflammatory markers (p > 0.05). | Vitamin D supplementation did not influence on CRP (std. mean differences −0.11; 95% CI −0.27–0.04; p = 0.15), TNF-α (std. mean differences −0.13; 95% CI −0.38–0.12; p = 0.31) and IL-6 concentrations (std. mean differences 0.1; 95% CI −0.43–0.63; p = 0.71). | Vitamin D supplementation significantly decreased the circulating hs-CRP level by 1.08 mg/L (95% CI, −2.13, −0.03), with the evidence of heterogeneity. Subgroup analysis suggested a higher reduction of 2.21 mg/L (95% CI, −3.50, −0.92) among participants with baseline hs-CRP level ≥5 mg/L. |
| Conclusions * | Vitamin D–calcium co-supplementation has beneficial effect on serum CRP concentrations. A beneficial effect was not seen for IL-6 and TNF-α concentrations. | Vitamin D supplementation may increase Treg/CD3 ratios in both healthy individuals and patients with autoimmune disorders and may increase Treg function. | Vitamin D supplementation reduced the rate of asthma exacerbation, especially in patients with vitamin D insufficiency. | In T2DM subjects, vitamin D supplementation is beneficial for the reduction in hs-CRP but does not have a significant influence on TNF-α and IL-6. | Vitamin D supplementation had no impact on serum CRP, IL10, and TNF-α, while significantly increased serum IL6. | Available high-quality RCTs did not support a beneficial effect of cholecalciferol on systemic IL-6 and CRP. | The use of vitamin D supplementation as a therapeutic or preventative measure for CVD is not supported by evidence. | Supplementation with vitamin D does not have a significant influence on changes in the concentration of selected inflammatory biomarkers in the obese and overweight subjects. | Vitamin D supplementation is beneficial for the reduction in circulating hs-CRP. |
| Pharmaceutical Drug | Dose | Follow-Up | Efficacy Yes | Efficacy No | Study | |
|---|---|---|---|---|---|---|
| Vitamin D1 e D2 | Paricalcitol | 400 IU day | 3 months | CRP | Mohsen Mazidi (2018) [15] | |
| Ergocalciferol | 50.000 IU/ month | 12 weeks–6 months | CRP | Mohsen Mazidi (2018) [15] | ||
| Vitamin D3 | Cholecalciferol | 200–6.000 IU/day 25.000–50.000 IU/week | 8–52 weeks | CRP | TNF-α e IL6 | Yanting Yu (2018) [14] |
| 400 IU/day–11,200 IU/day | 4 weeks–12 months | IL6 | CRP, IL10 e TNF-α | Mohsen Mazidi (2018) [15] | ||
| 4000 IU/week | 8 weeks | FMD *, CRP, IL-6 e TNF-α | Tari Agbalalah (2017) [17] | |||
| 4000 IU/day | 24 weeks | hs-CRP ** | Neng Chen (2014) [19] | |||
| ≤4000 IU/day | >12 weeks | CRP | TNF-α e IL6 | Yanting Yu (2018) [14] | ||
| Scheme 2019. | Maryam Safabakhsh (2019) [20] | Sedagh Jafamejad (2018) [21] | Ammar W. Ashor (2015) [22] | Ammar W. Ashor (2014) [23] |
|---|---|---|---|---|
| Databases searched | PubMed, Scopus, ISI Web of Science e Google Scholar | Scopus, Cochrane Library, PubMed and Google Scholar | MEDLINE, Embase, Cochrane Library and Scopus | Medline, Embase, Cochrane Library, and Scopus |
| Articles included | 11 | 12 | 46 | 44 |
| Type of patients analyzed | Diabetic subjects/Nonsmokers | Patients with chronic diseases | Adult participants >18 years | Adult participants |
| Posology | 500 mg/day | 250 mg/day–1 g/day | 500–2000 mg/day | 500 mg/day–1 g/day |
| Intervention duration range | 1 day–8 weeks | 4–24 weeks | 4–52 weeks | 1 day–8 weeks |
| Endpoint | The effect of vitamin C on reducing CRP or hs-CRP level. | The effects of supplementation with vitamin C on serum C-reactive Protein (CRP) levels. | The effects of antioxidant vitamins C and E supplementation on endothelial function. | The effect of supplementation with vitamin C on endothelial function. |
| Results | Vitamin C could decrease CRP levels relative to placebo group ([WMD] = −0.73 mg/L: 95% CI: −1.30 to −0.15, p = 0.013). | Supplementation with vitamin C significantly lowered CRP among trials. | Significant improvements in endothelial function were observed in trials supplementing with vitamin C (500–2000 mg/d) (SMD: 0·25, 95% CI 0·02, 0·49, P¼0·043) | A beneficial effect of vitamin C on endothelial function was found (SMD: 0.50, 95% CI: 0.34, 0.66, p < 0.001) |
| Conclusions | Vitamin C supplementation might have a significant effect only on CRP reduction. | Vitamin C supplementation reduces serum CRP levels. | Supplementation with vitamin C improves endothelial function. | Supplementation with vitamin C improved endothelial function. |
| Administration | Dose | Follow-Up | Endpoint | Efficacy | Study |
|---|---|---|---|---|---|
| Intravenous | 250 mg/day | 8 weeks | CPR | Yes | Biniaz 2014 [24] |
| 300 mg/day | 24 weeks | CPR | Yes | Attallah 2006 [25] | |
| Oral | 1 g/day | 10 days | EF * | Yes | De Marchi 2012 [26] |
| 1 g/day | 4 days | CRP | Yes | Colby 2011 [27] | |
| 1 g/day | 4 weeks | CRP | Yes | Modi 2014 [28] | |
| 2 g/day | 4 weeks | EF * | Yes | Antoniades 2004 [29] Tousoulis 2007 [30] |
| Zarezadeh M (2019) [31] | Akbari M (2018) [32] | |
|---|---|---|
| Databases searched | SCOPUS, PubMed, Cochrane Library, Embase, Google Scholar | Cochrane Library, EMBASE, PubMed, and Web of Science |
| Articles included | 13 | 6 |
| Type of patients analyzed | Patients with chronic diseases | Patients with metabolic syndrome |
| Posology | 3 to 25 mg/day | 6 to 10 mg/day |
| Intervention duration range | From 4 to 60 weeks | From 4 weeks to 14 months |
| Endpoint | To evaluate the effect of supplementation with melatonin on inflammatory biomarker levels | To evaluate the effect of supplementation with melatonin on inflammatory markers among subjects with MetS or related disorders. |
| Results | Melatonin supplementation significantly decreased TNF-α and IL-6 levels [(WMD = −2.24 pg/mL; 95% CI −3.45, −1.03; p < 0.001; I2 = 96.7%, Pheterogeneity < 0.001) and (WMD = −30.25 pg/mL; 95% CI −41.45, −19.06; p < 0.001, 2I = 99.0%; Pheterogeneity < 0.001)], respectively. The effect of melatonin on CRP levels was marginal. | Melatonin supplementation significantly reduced C-reactive protein (SMD = −1.80; 95% CI −3.27, −0.32; p = 0.01; I2: 95.2) and interleukin 6 (IL-6) concentrations (SMD= −2.02; 95% CI −3.57, −0.47; p = 0.01; I2: 91.2) among patients with MetS and related disorders; however, it did not affect TNF-α concentrations. |
| Conclusions | Melatonin supplementation significantly reduced TNF-α and IL-6 levels. The supplementation with melatonin improved the levels of TNF-α and IL-6 more efficiently in studies, which were conducted for ≥ 12 weeks and at a dosage ≥ 10 mg/day. | The promising effect of melatonin administration on reducing CRP and IL-6 among patients with metabolic syndrome and related disorders. |
| Administration | Dose | Follow-Up | Endpoint | Efficacy | Study |
|---|---|---|---|---|---|
| Oral | 25 mg/day | 26 weeks | TNF and IL-6 | Yes | SanchezLopez A (2018) [33] |
| 20 mg/day | 12 weeks | TNF | Yes | Lissoni P (1996) [34] | |
| 10 mg/day | 12 weeks 12 weeks 4 weeks | CPR | Yes | Raygan et al. (2017) [35] Pakravan (2017) [36] Javanmard (2016) [37] | |
| 26 weeks 60 weeks 4 weeks | TNF and IL-6 | Yes | Forest CM (2007) [38] Celinski et al. (2014) [39] Cichoz-Lach et al. (2010) [40] | ||
| 6 mg/day | 6 weeks | TNF and IL-6 | Yes | Mesri Alamdari (2015) [41] | |
| 5 mg/day | 52 weeks | CPR | Yes | Chojnacki C (2011) [42] |
| Mousavi SM (2018) [43] | |
|---|---|
| Databases searched | PubMed, SCOPUS, and Google Scholar |
| Articles included | 8 |
| Type of patients analyzed | Hemodialysis patients |
| Posology | 50 mg/day |
| Intervention duration range | 6–25 weeks |
| Endpoint | Effect of supplementation with zinc on plasma CRP concentrations in adults |
| Results | The results of the meta-analysis displayed a significant reduction in circulating CRP levels (WMD: −1.68 mg/L; 95% CI: −2.4 to −0.9, p =< 0.001) following supplementation with zinc. |
| Conclusions | Supplementation with zinc markedly reduced plasma CRP concentration |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrao, S.; Mallaci Bocchio, R.; Lo Monaco, M.; Natoli, G.; Cavezzi, A.; Troiani, E.; Argano, C. Does Evidence Exist to Blunt Inflammatory Response by Nutraceutical Supplementation during COVID-19 Pandemic? An Overview of Systematic Reviews of Vitamin D, Vitamin C, Melatonin, and Zinc. Nutrients 2021, 13, 1261. https://doi.org/10.3390/nu13041261
Corrao S, Mallaci Bocchio R, Lo Monaco M, Natoli G, Cavezzi A, Troiani E, Argano C. Does Evidence Exist to Blunt Inflammatory Response by Nutraceutical Supplementation during COVID-19 Pandemic? An Overview of Systematic Reviews of Vitamin D, Vitamin C, Melatonin, and Zinc. Nutrients. 2021; 13(4):1261. https://doi.org/10.3390/nu13041261
Chicago/Turabian StyleCorrao, Salvatore, Raffaella Mallaci Bocchio, Marika Lo Monaco, Giuseppe Natoli, Attilio Cavezzi, Emidio Troiani, and Christiano Argano. 2021. "Does Evidence Exist to Blunt Inflammatory Response by Nutraceutical Supplementation during COVID-19 Pandemic? An Overview of Systematic Reviews of Vitamin D, Vitamin C, Melatonin, and Zinc" Nutrients 13, no. 4: 1261. https://doi.org/10.3390/nu13041261
APA StyleCorrao, S., Mallaci Bocchio, R., Lo Monaco, M., Natoli, G., Cavezzi, A., Troiani, E., & Argano, C. (2021). Does Evidence Exist to Blunt Inflammatory Response by Nutraceutical Supplementation during COVID-19 Pandemic? An Overview of Systematic Reviews of Vitamin D, Vitamin C, Melatonin, and Zinc. Nutrients, 13(4), 1261. https://doi.org/10.3390/nu13041261









