Potential Clinical Applications of Pro-Resolving Lipids Mediators from Docosahexaenoic Acid
Abstract
:1. Introduction
2. Methods
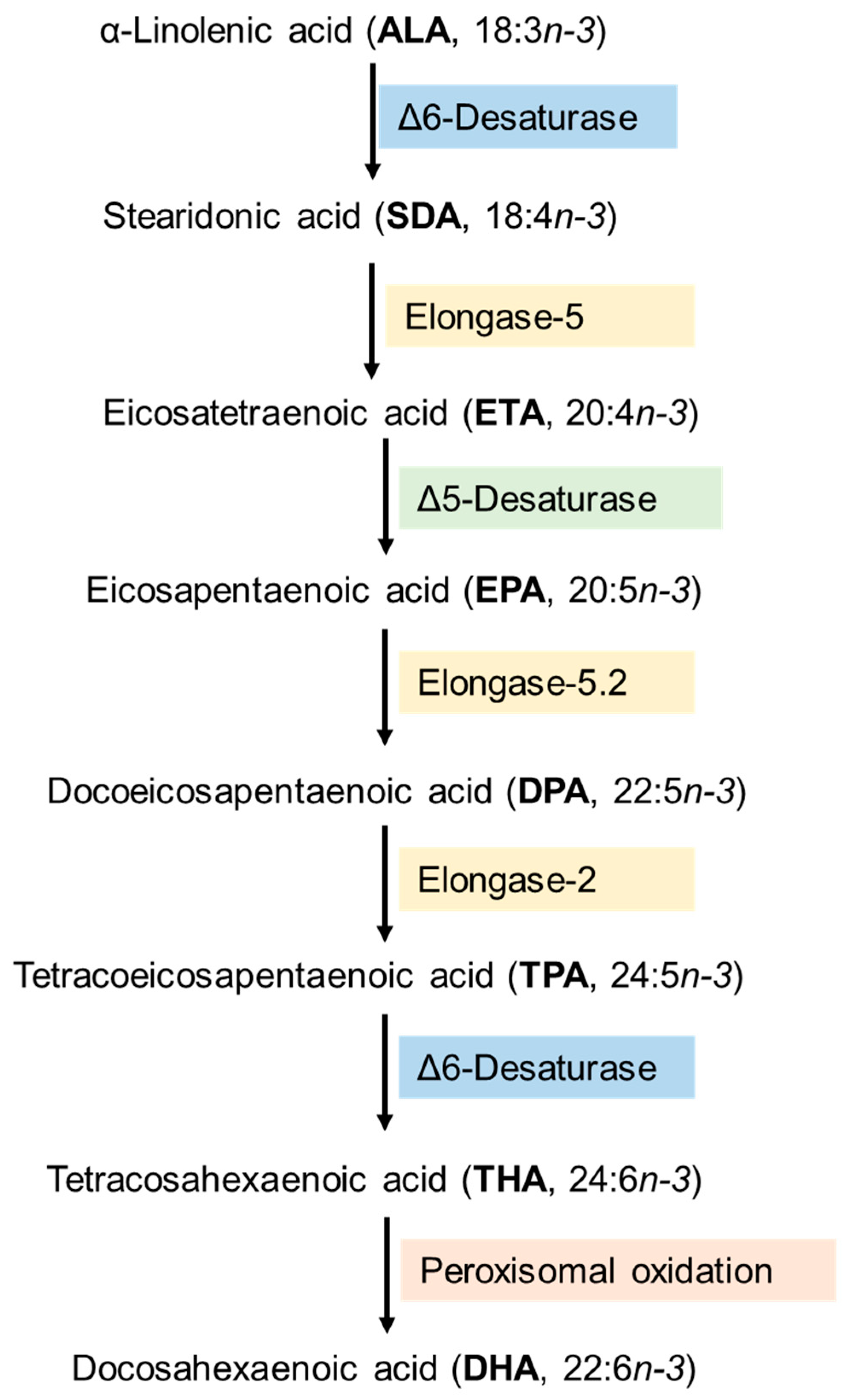
3. Synthesis and Metabolism of DHA-Derived Lipid Mediators
4. SPMs and Regulation of Inflammatory Processes
5. Neurodegenerative Diseases
5.1. Alzheimer Disease (AD)
5.2. Parkinson Disease (PD)
6. Respiratory Diseases
7. Metabolic Syndrome
8. Cardiovascular Diseases
9. Liver Diseases
10. Other Pathologies
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petermann, A.B.; Reyna-Jeldes, M.; Ortega, L.; Coddou, C.; Yévenes, G.E. Roles of the unsaturated fatty acid docosahexaenoic acid in the central nervous system: Molecular and cellular insights. Int. J. Mol. Sci. 2022, 23, 5390. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Docosahexaenoic acid. Ann. Nutr. Metab. 2016, 69, 8–21. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, I.; Falcato, F.; Bandarra, N.; Rauter, A.P. Resolvins, protectins, and maresins: DHA-derived specialized pro-resolving mediators, biosynthetic pathways, synthetic approaches, and their role in inflammation. Molecules 2022, 27, 1677. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Fard, S.; Wang, F.; Sinclair, A.J.; Elliott, G.; Turchini, G.M. How does high DHA fish oil affect health? A systematic review of evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 1684–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Naidu, K.A.; Shang, X.; Keum, Y.S. Omega−3 polyunsaturated fatty acids (PUFAs): Emerging plant and microbial sources, oxidative stability, bioavailability, and health benefits—A review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Kothapalli, K.S.; Brenna, J.T. Desaturase and elongase limiting endogenous long chain polyunsaturated fatty acid biosynthesis. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 103–110. [Google Scholar] [CrossRef]
- Punia, S.; Sandhu, K.S.; Siroha, A.K.; Dhull, S.B. Omega 3-metabolism, absorption, bioavailability and health benefits–A review. Pharmanutrition 2019, 10, 100162. [Google Scholar] [CrossRef]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef]
- Harris, M.A.; Reece, M.S.; McGregor, J.A.; Wilson, J.W.; Burke, S.M.; Wheeler, M.; Anderson, J.E.; Auld, G.W.; French, J.I.; Allen, K.G.D. The effect of omega-3 docosahexaenoic acid supplementation on gestational length: Randomized trial of supplementation compared to nutrition education for increasing n-3 intake from foods. Biomed Res. Int. 2015, 2015, 123078. [Google Scholar] [CrossRef] [Green Version]
- Drover, J.R.; Hoffman, D.R.; Castañeda, Y.S.; Morale, S.E.; Garfield, S.; Wheaton, D.H.; Birch, E.E. Cognitive function in 18-month-old term infants of the DIAMOND study: A randomized, controlled clinical trial with multiple dietary levels of docosahexaenoic acid. Early Hum. Dev. 2011, 87, 223–230. [Google Scholar] [CrossRef]
- Willatts, P.; Forsyth, S.; Agostoni, C.; Casaer, P.; Riva, E.; Boehm, G. Effects of long-chain PUFA supplementation in infant formula on cognitive function in later childhood. Am. J. Clin. Nutr. 2013, 98, 536S–542S. [Google Scholar] [CrossRef] [Green Version]
- Asztalos, I.B.; Gleason, J.A.; Sever, S.; Gedik, R.; Asztalos, B.F.; Horvath, K.V.; Dansinger, M.L.; Lamon-Fava, S.; Schaefer, E.J. Effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular disease risk factors: A randomized clinical trial. Metabolism 2016, 65, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.; Couture, P.; Leclerc, M.; Charest, A.; Marin, J.; Marie-claude, L.; Talbot, D.; Tchernof, A.; Lamarche, B. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: The Comparing EPA to DHA (ComparED) Study. Am. J. Clin. Nutr. 2016, 104, 280–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stonehouse, W.; Conlon, C.A.; Podd, J.; Hill, S.R.; Minihane, A.M.; Haskell, C.; Kennedy, D. DHA supplementation improved both memory and reaction time in healthy young adults: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 1134–1143. [Google Scholar] [CrossRef] [Green Version]
- Christen, W.G.; Schaumberg, D.A.; Glynn, R.J.; Buring, J.E. Dietary ω-3 fatty acid and fish intake and incident age-related macular degeneration in women. Arch. Ophthalmol. 2011, 129, 921–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.D.; Warnke, I.; Weimann, A.; Calder, P.C. Expert opinion on benefits of long-chain omega-3 fatty acids (DHA and EPA) in aging and clinical nutrition. Nutrients 2020, 12, 2555. [Google Scholar] [CrossRef]
- Kuda, O. Bioactive metabolites of docosahexaenoic acid. Biochimie 2017, 136, 12–20. [Google Scholar] [CrossRef]
- Valente, M.; Dentoni, M.; Bellizzi, F.; Kuris, F.; Gigli, G.L. Specialized pro-resolving mediators in neuroinflammation: Overview of studies and perspectives of clinical applications. Molecules 2022, 27, 4836. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; Souza, F.d.C.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef]
- Dasilva, G.; Lois, S.; Méndez, L.; Miralles-Pérez, B.; Romeu, M.; Ramos-Romero, S.; Torres, J.L.; Medina, I. Fish oil improves pathway-oriented profiling of lipid mediators for maintaining metabolic homeostasis in adIpose tissue of prediabetic rats. Front. Immunol. 2021, 12, 608875. [Google Scholar] [CrossRef] [PubMed]
- Hayford, F.E.; Ozturk, M.; Dolman, R.C.; Blaauw, R.; Nienaber, A.; Loots, D.T.; Brombacher, F.; Smuts, C.M.; Parihar, S.P.; Malan, L. Longer-term omega-3 LCPUFA more effective adjunct therapy for tuberculosis than ibuprofen in a C3HeB/FeJ tuberculosis mouse model. Front. Immunol. 2021, 12, 659943. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Serhan, C.N. Specialized pro-resolving mediator network: An update on production and actions. Essays Biochem. 2020, 64, 443–462. [Google Scholar] [PubMed]
- Gallo, C.G.; Fiorino, S.; Posabella, G.; Antonacci, D.; Tropeano, A.; Pausini, E.; Pausini, C.; Guarniero, T.; Hong, W.; Giampieri, E.; et al. The function of specialized pro-resolving endogenous lipid mediators, vitamins, and other micronutrients in the control of the inflammatory processes: Possible role in patients with SARS-CoV-2 related infection. Prostaglandins Other Lipid Mediat. 2022, 159, 106619. [Google Scholar] [CrossRef]
- Kotlęga, D.; Peda, B.; Drozd, A.; Zembroń-Łacny, A.; Stachowska, E.; Gramacki, J.; Szczuko, M. Prostaglandin E2, 9S-, 13S-HODE and resolvin D1 are strongly associated with the post-stroke cognitive impairment. Prostaglandins Other Lipid Mediat. 2021, 156, 106576. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, X.; Hjorth, E.; Colas, R.A.; Schroeder, L.; Granholm, A.-C.; Serhan, C.N.; Schultzberg, M. Pro-resolving lipid mediators improve neuronal survival and increase Aβ42 phagocytosis. Mol. Neurobiol. 2016, 53, 2733–2749. [Google Scholar] [CrossRef] [Green Version]
- Dalli, J.; Colas, R.A.; Quintana, C.; Barragan-Bradford, D.; Hurwitz, S.; Levy, B.D.; Choi, A.M.; Serhan, C.N.; Baron, R.M. Human sepsis eicosanoid and pro-resolving lipid mediator temporal profiles: Correlations with survival and clinical outcomes. Crit. Care Med. 2017, 45, 58–68. [Google Scholar] [CrossRef]
- Wang, C.-W.; Colas, R.A.; Dalli, J.P.; Arnardottir, H.H.; Nguyen, D.; Hasturk, H.; Chiang, N.; Van Dyke, T.E.; Serhan, C.N. Maresin 1 biosynthesis and proresolving anti-infective functions with human-localized aggressive periodontitis leukocytes. Infect. Immun. 2015, 84, 658–665. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, M.D.; Patel, J.; Staton, K.; Martindale, R.G.; Moore, F.A.; Upchurch, G.R. Can specialized pro-resolving mediators deliver benefit originally expected from fish oil? Curr. Gastroenterol. Rep. 2018, 20, 40. [Google Scholar] [CrossRef]
- Leslie, C.C. Cytosolic phospholipase A2: Physiological function and role in disease. J. Lipid Res. 2015, 56, 1386–1402. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N. Novel pro-resolving lipid mediators in inflammation are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harwood, J.L. Polyunsaturated fatty acids: Conversion to lipid mediators, roles in inflammatory diseases and dietary sources. Int. J. Mol. Sci. 2023, 24, 8838. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Serhan, C.N. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol. Asp. Med. 2017, 58, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation-resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef] [Green Version]
- Rouzer, C.A.; Marnett, L.J. Cycloxygenases: Structural and functional insights. J. Lipid Res. 2009, 50, S29–S34. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 2015, 1851, 397–413. [Google Scholar] [CrossRef] [Green Version]
- Balas, L.; Guichardant, M.; Durand, T.; Lagarde, M. Confusion between protectin D1 (PD1) and its isomer protectin DX (PDX). An overview on the dihydroxy-docosatrienes described to date. Biochimie 2014, 99, 1–7. [Google Scholar] [CrossRef]
- Serhan, C.N.; Dalli, J.; Karamnov, S.; Choi, A.; Park, C.-K.; Xu, Z.-Z.; Ji, R.-R.; Zhu, M.; Petasis, N.A. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012, 26, 1755–1765. [Google Scholar] [CrossRef] [Green Version]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Aukema, H.M. Advances in our understanding of oxylipins derived from dietary PUFAs. Am. Soc. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wu, X.; Liu, S.; Shen, D.; Zhu, J.; Liu, K. Role of resolvins in the inflammatory resolution of neurological diseases. Front. Pharmacol. 2020, 11, 612. [Google Scholar] [CrossRef]
- Han, Y.-H.; Lee, K.; Saha, A.; Han, J.; Choi, H.; Noh, M.; Lee, Y.-H.; Lee, M.-O. Specialized proresolving mediators for therapeutic interventions targeting metabolic and inflammatory disorders. Biomol. Ther. 2021, 29, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Ariyoshi, T.; Hagihara, M.; Eguchi, S.; Fukuda, A.; Iwasaki, K.; Oka, K.; Takahashi, M.; Yamagishi, Y.; Mikamo, H. Clostridium butyricum MIYAIRI 588-induced protectin D1 has an anti-inflammatory effect on antibiotic-induced intestinal disorder. Front. Microbiol. 2020, 11, 587725. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Yacoubian, S.; Lee, C.-H.; Yang, R.; Petasis, N.A.; Serhan, C.N. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Pirault, J.; Bäck, M. Lipoxin and resolvin receptors transducing the resolution of inflammation in cardiovascular disease. Front. Pharmacol. 2018, 9, 1273. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.-H.; Kim, H.-J.; Na, H.; Nam, M.-W.; Kim, J.-Y.; Kim, J.-S.; Koo, S.-H.; Lee, M.-O. RORα induces KLF4-mediated M2 polarization in the liver macrophages that protect against nonalcoholic steatohepatitis. Cell Rep. 2017, 20, 124–135. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Takaishi, S.; Nagasaki, M.; Onozawa, Y.; Iino, I.; Maeda, H.; Komai, T.; Oda, T. Medium-chain fatty acid-sensing receptor, GPR84, is a proinflammatory receptor. J. Biol. Chem. 2013, 288, 10684–10691. [Google Scholar] [CrossRef] [Green Version]
- Liang, P.; Henning, S.M.; Guan, J.; Grogan, T.; Elashoff, D.; Olefsky, J.M.; Cohen, P.; Aronson, W.J. Role of host GPR120 in mediating dietary omega-3 fatty acid inhibition of prostate cancer. J. Natl. Cancer Inst. 2019, 111, 52–59. [Google Scholar] [CrossRef]
- Duffney, P.F.; Falsetta, M.L.; Rackow, A.R.; Thatcher, T.H.; Phipps, R.P.; Sime, P.J. Key roles for lipid mediators in the adaptive immune response. J. Clin. Investig. 2018, 128, 2724–2731. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [Green Version]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef]
- Beegun, I.; Koenis, D.S.; Alusi, G.; Dalli, J. Dysregulated maresin concentrations in plasma and nasal secretions from patients with chronic rhinosinusitis. Front. Immunol. 2021, 12, 733019. [Google Scholar] [CrossRef]
- Giera, M.; Ioan-Facsinay, A.; Toes, R.; Gao, F.; Dalli, J.; Deelder, A.M.; Serhan, C.N.; Mayboroda, O.A. Lipid lipid mediator profiling of human synovial fluid in rheumatoid arthritis patients by means of LC-MS/MS. Biochim. Biophys. Acta 2012, 1821, 1415–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poisson, L.M.; Suhail, H.; Singh, J.; Datta, I.; Denic, A.; Labuzek, K.; Hoda, M.N.; Shankar, A.; Kumar, A.; Cerghet, M.; et al. Untargeted plasma metabolomics identifies endogenous metabolite with drug-like properties in chronic animal model of multiple sclerosis. J. Biol. Chem. 2015, 290, 30697–30712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N.; Chiang, N.; Dalli, J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015, 27, 200–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N. Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol. Asp. Med. 2017, 58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xu, G.; Newton, P.T.; Chagin, A.S.; Mkrtchian, S.; Carlström, M.; Zhang, X.M.; Harris, R.A.; Cooter, M.; Berger, M.; et al. Maresin 1 attenuates neuroinflammation in a mouse model of perioperative neurocognitive disorders. Br. J. Anaesth. 2019, 122, 350–360. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.S.; Conte, M.S. Specialized pro-resolving lipid mediators in cardiovascular disease, diagnosis, and therapy. Adv. Drug Deliv. Rev. 2020, m159, 170–179. [Google Scholar] [CrossRef]
- Recchiuti, A.; Mattoscio, D.; Isopi, E. Roles, actions, and therapeutic potential of specialized pro-resolving lipid mediators for the treatment of inflammation in cystic fibrosis. Front. Pharmacol. 2019, 10, 252. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Chiang, N.; Dalli, J. New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol. Asp. Med. 2018, 64, 1–17. [Google Scholar] [CrossRef]
- Fredman, G.; Ozcan, L.; Spolitu, S.; Hellmann, J.; Spite, M.; Backs, J.; Tabas, I. Resolvin D1 limits 5-lipoxygenase nuclear localization and leukotriene B4 synthesis by inhibiting a calciumactivated kinase pathway. Proc. Natl. Acad. Sci. USA 2014, 111, 14530–14535. [Google Scholar] [CrossRef]
- Motwani, M.P.; Colas, R.A.; George, M.J.; Flint, J.D.; Dalli, J.; Richard-Loendt, A.; De Maeyer, R.P.; Serhan, C.N.; Gilroy, D.W. Pro-resolving mediators promote resolution in a human skin model of UV-killed Escherichia coli-driven acute inflammation. JCI Insight 2018, 3, e94463. [Google Scholar] [CrossRef] [Green Version]
- Werz, O.; Gerstmeier, J.; Libreros, S.; De la Rosa, X.; Werner, M.; Norris, P.C.; Chiang, N.; Serhan, C.N. Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat. Commun. 2018, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panigrahy, D.; Gilligan, M.M.; Serhan, C.N.; Kashfi, K. Resolution of inflammation: An organizing principle in biology and medicine. Pharmacol. Ther. 2021, 227, 107879. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.J.; Heo, S.Y.; Ju, J.H.; Oh, B.R.; Son, W.S.; Seo, J.W. Synthesis of two new lipid mediators from docosahexaenoic acid by combinatorial catalysis involving enzymatic and chemical reaction. Sci. Rep. 2020, 10, 18849. [Google Scholar] [CrossRef]
- Schebb, N.H.; Kühn, H.; Kahnt, A.S.; Rund, K.M.; O’Donnell, V.B.; Flamand, N.; Peters-Golden, M.; Jakobsson, P.J.; Weylandt, K.H.; Rohwer, N.; et al. Formation, Signaling and Occurrence of specialized pro-resolving lipid mediators—What is the evidence so far? Front. Pharmacol. 2022, 13, 838782. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R. Recent advances in the mechanisms of neuroinflammation and their roles in neurodegeneration. Neurochem. Int. 2018, 120, 13–20. [Google Scholar] [CrossRef]
- Raj, D.; Yin, Z.; Breur, M.; Doorduin, J.; Holtman, I.R.; Olah, M.; Mantingh-Otter, I.J.; Van Dam, D.; De Deyn, P.P.; Dunnen, W.D.; et al. Increased white matter inflammation in aging- and Alzheimer’s disease brain. Front. Mol. Neurosci. 2017, 10, 206. [Google Scholar] [CrossRef] [Green Version]
- Newcombe, E.A.; Camats-Perna, J.; Silva, M.L.; Valmas, N.; Huat, T.J.; Medeiros, R. Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease. J. Neuroinflammation 2018, 15, 276. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhu, M.; Hjorth, E.; Cortés-Toro, V.; Eyjolfsdottir, H.; Graff, C.; Nennesmo, I.; Palmblad, J.; Eriksdotter, M.; Sambamurti, K.; et al. Resolution of inflammation is altered in Alzheimer’s disease. Alzheimers Dement 2015, 11, 40–50.e2. [Google Scholar] [CrossRef] [Green Version]
- Do, K.V.; Hjorth, E.; Wang, Y.; Jun, B.; Kautzmann, M.A.; Ohshima, M.; Eriksdotter, M.; Schultzberg, M.; Bazan, N.G. Cerebrospinal fluid profile of lipid mediators in alzheimer’s disease. Cell Mol. Neurobiol. 2023, 43, 797–811. [Google Scholar] [CrossRef]
- Mizwicki, M.T.; Liu, G.; Fiala, M.; Magpantay, L.; Sayre, J.; Siani, A.; Mahanian, M.; Weitzman, R.; Hayden, E.Y.; Rosenthal, M.J.; et al. 1α,25-Dihydroxyvitamin D3 and resolvin D1 retune the balance between amyloid-β phagocytosis and inflammation in Alzheimer’s disease patients. J. Alzheimer’s Dis. 2013, 34, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Leppert, A.; Tan, S.; van der Gaag, B.; Li, N.; Schultzberg, M.; Hjorth, E. Maresin 1 attenuates pro-inflammatory activation induced by β-amyloid and stimulates its uptake. J. Cell Mol. Med. 2021, 25, 434–447. [Google Scholar] [CrossRef]
- Emre, C.; Arroyo-García, L.E.; Do, K.V.; Jun, B.; Ohshima, M.; Alcalde, S.G.; Cothern, M.L.; Maioli, S.; Nilsson, P.; Hjorth, E.; et al. Intranasal delivery of pro-resolving lipid mediators rescues memory and gamma oscillation impairment in App NL-G-F/NL-G-F mice. Commun. Biol. 2022, 5, 245. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Wang, X.; Wang, S.; Wei, Y.; Feng, J.; Zhu, M. Maresin 1 Improves Cognitive Decline and Ameliorates Inflammation in a Mouse Model of Alzheimer’s Disease. Front. Cell Neurosci. 2019, 13, 466. [Google Scholar] [CrossRef] [Green Version]
- Kantarci, A.; Aytan, N.; Palaska, I.; Stephens, D.; Crabtree, L.; Benincasa, C.; Jenkins, B.G.; Carreras, I.; Dedeoglu, A. Combined administration of resolvin E1 and lipoxin A4 resolves inflammation in a murine model of Alzheimer’s disease. Exp. Neurol. 2018, 300, 111–120. [Google Scholar] [CrossRef]
- Salari, M.; Mirmosayyeb, O.; Etemadifar, M.; Shaygannejad, V.; Khorvash, F.; Najafi, M.R.; Ashtari, F.; Chitsaz, A. Demographic features and clinical characteristics of patients with Parkinson’s disease in Isfahan, Iran. Iran J. Neurol. 2018, 17, 6–10. [Google Scholar]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Krashia, P.; Cordella, A.; Nobili, A.; La Barbera, L.; Federici, M.; Leuti, A.; Campanelli, F.; Natale, G.; Marino, G.; Calabrese, V.; et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat. Commun. 2019, 10, 3945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadeem, H.; Zhou, B.; Goldman, D.; Romley, J. Association between use of ß2-adrenergic receptor agonists and incidence of Parkinson’s disease: Retrospective cohort analysis. PLoS ONE 2022, 17, e0276368. [Google Scholar] [CrossRef]
- La Barbera, L.; Nobili, A.; Cauzzi, E.; Paoletti, I.; Federici, M.; Saba, L.; Giacomet, C.; Marino, R.; Krashia, P.; Melone, M.; et al. Upregulation of Ca2+-binding proteins contributes to VTA dopamine neuron survival in the early phases of Alzheimer’s disease in Tg2576 mice. Mol. Neurodegener. 2022, 17, 76. [Google Scholar] [CrossRef]
- Xu, M.X.; Tan, B.C.; Zhou, W.; Wei, T.; Lai, W.H.; Tan, J.W.; Dong, J.-H. Resolvin D1, an endogenous lipid mediator for inactivation of inflammation-related signaling pathways in microglial cells, prevents lipopolysaccharide-induced inflammatory responses. CNS Neurosci. Ther. 2013, 19, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gao, X.; Yang, C.; Chen, L.; Chen, Z. Resolvin D1 attenuates Mpp+-induced Parkinson disease via inhibiting inflammation in PC12 cells. Med. Sci. Monit. 2017, 23, 2684–2691. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Zhang, Y.; Zhang, R.; Qiao, S.; Fan, J. Resolvin D2 recovers neural injury by suppressing inflammatory mediator’s expression in lipopolysaccharide-induced Parkinson’s disease rat model. Biochem. Biophys. Res. Commun. 2015, 460, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Calandria, J.M.; Sharp, M.W.; Bazan, N.G. The Docosanoid neuroprotectin D1 induces TH-positive neuronal survival in a cellular model of Parkinson’s disease. Cell Mol. Neurobiol. 2015, 35, 1127–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Fernández, A.; Zandee, S.; Mastrogiovanni, M.; Charabati, M.; Rubbo, H.; Prat, A.; López-Vales, R. Administration of maresin-1 ameliorates the physiopathology of experimental autoimmune encephalomyelitis. J. Neuroinflammation 2022, 19, 27. [Google Scholar] [CrossRef]
- Kooij, G.; Troletti, C.D.; Leuti, A.; Norris, P.C.; Riley, I.; Albanese, M.; Ruggieri, S.; Libreros, S.; van der Pol, S.M.; Hof, B.v.H.; et al. Specialized pro-resolving lipid mediators are differentially altered in peripheral blood of patients with multiple sclerosis and attenuate monocyte and blood-brain barrier dysfunction. Haematologica 2020, 105, 2056–2070. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Fiala, M.; Mizwicki, M.T.; Sayre, J.; Magpantay, L.; Siani, A.; Mahanian, M.; Chattopadhyay, M.; La Cava, A.; Wiedau-Pazos, M. Neuronal phagocytosis by inflammatory macrophages in ALS spinal cord: Inhibition of inflammation by resolvin D1. Am. J. Neurodegener. Dis. 2012, 1, 60–74. [Google Scholar]
- Zúñiga-Hernández, J.; Sambra, V.; Echeverría, F.; Videla, L.A.; Valenzuela, R. N-3 PUFAs and their specialized pro-resolving lipid mediators on airway inflammatory response: Beneficial effects in the prevention and treatment of respiratory diseases. Food Funct. 2022, 13, 4260–4272. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, W.; Wang, X.; Zhang, C.; Cai, J.; Yu, S.; Sun, J.; Tian, Z. SGK1 enhances Th9 cell differentiation and airway inflammation through NF-κB signaling pathway in asthma. Cell Tissue Res. 2020, 382, 563–574. [Google Scholar] [CrossRef]
- Feng, X.; Yang, Y.; Zheng, Y.; Song, J.; Hu, Y.; Xu, F. Effects of catalpol on asthma by airway remodeling via inhibiting TGF-β1 and EGF in ovalbumin-induced asthmatic mice. Am. J. Transl. Res. 2020, 12, 4084–4093. [Google Scholar]
- Johnson, R.K.; Manke, J.; Campbell, M.; Armstrong, M.; Boorgula, M.P.; Pinheiro, G.; Santana, C.V.N.; Mathias, R.A.; Barnes, K.C.; Cruz, A.; et al. Lipid mediators are detectable in the nasal epithelium and differ by asthma status in female subjects. J. Allergy Clin. Immunol. 2022, 150, 965–971.e8. [Google Scholar] [CrossRef] [PubMed]
- Miyata, J.; Fukunaga, K.; Iwamoto, R.; Isobe, Y.; Niimi, K.; Takamiya, R.; Takihara, T.; Tomomatsu, K.; Suzuki, Y.; Oguma, T.; et al. Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. J. Allergy Clin. Immunol. 2013, 131, 353–360.e2. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.X.; Shi, Z.A.; Ou, G.C.; Chen, X.J.; Liu, Q.; Zeng, D.; Nie, X.J.; Chen, J.J. Maresin-2 alleviates allergic airway inflammation in mice by inhibiting the activation of NLRP3 inflammasome, Th2 type immune response and oxidative stress. Mol. Immunol. 2022, 146, 78–86. [Google Scholar] [CrossRef]
- Ou, G.; Liu, Q.; Yu, C.; Chen, X.; Zhang, W.; Chen, Y.; Wang, T.; Luo, Y.; Jiang, G.; Zhu, M.; et al. The protective effects of maresin 1 in the OVA-induced asthma mouse model. Mediat. Inflamm. 2021, 14, 13035–13040. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, N.; Burkett, P.R.; Dalli, J.; Abdulnour, R.-E.E.; Colas, R.; Ramon, S.; Phipps, R.P.; Petasis, N.A.; Kuchroo, V.K.; Serhan, C.N.; et al. Maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J. Immunol. 2015, 194, 863–867. [Google Scholar] [CrossRef] [Green Version]
- Miyata, J.; Arita, M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol. Int. 2015, 64, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Saheb Sharif-Askari, N.; Soares, N.C.; Mohamed, H.A.; Saheb Sharif-Askari, F.; Alsayed, H.A.; Al-Hroub, H.; Salameh, L.; Osman, R.S.; Mahboub, B.; Hamid, Q.; et al. Saliva metabolomic profile of COVID-19 patients associates with disease severity. Metabolomics. 2022, 18, 81. [Google Scholar] [CrossRef]
- Regidor, P.A.; de la Rosa, X.; Santos, F.G.; Rizo, J.M.; Banzo, R.G.; Silva, R.S. Acute severe SARS COVID-19 patients produce pro-resolving lipids mediators and eicosanoids. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6782–6796. [Google Scholar]
- Schwarz, B.; Sharma, L.; Roberts, L.; Peng, X.; Bermejo, S.; Leighton, I.; Casanovas-Massana, A.; Minasyan, M.; Farhadian, S.; Ko, A.I.; et al. Cutting edge: Severe SARS-CoV-2 infection in humans is defined by a shift in the serum lipidome, resulting in dysregulation of eicosanoid immune mediators. J. Immunol. 2021, 206, 329–334. [Google Scholar] [CrossRef]
- Alonso, D.; Balsa, J.; Barbero, J.; Hernández, G. Neumonía vírica. Neumonía en la COVID-19. Medicine 2022, 13, 3224–3234. [Google Scholar]
- Kumar, V. Pulmonary innate immune response determines the outcome of inflammation during pneumonia and sepsis-associated acute lung injury. Front. Immunol. 2020, 11, 1722. [Google Scholar] [CrossRef]
- Abdulnour, R.E.; Sham, H.P.; Douda, D.N.; Colas, R.A.; Dalli, J.; Bai, Y.; Ai, X.; Serhan, C.N.; Levy, B.D. Aspirin-triggered resolvin D1 is produced during self-resolving gram-negative bacterial pneumonia and regulates host immune responses for the resolution of lung inflammation. Mucosal. Immunol. 2016, 9, 1278–1287. [Google Scholar] [CrossRef] [Green Version]
- Codagnone, M.; Cianci, E.; Lamolinara, A.; Mari, V.C.; Nespoli, A.; Isopi, E.; Mattoscio, D.; Arita, M.; Bragonzi, A.; Iezzi, M.; et al. Resolvin D1 enhances the resolution of lung inflammation caused by long-term Pseudomonas aeruginosa infection. Mucosal. Immunol. 2018, 11, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Croasdell, A.; Lacy, S.H.; Thatcher, T.H.; Sime, P.J.; Phipps, R.P. Resolvin D1 dampens pulmonary inflammation and promotes clearance of Nontypeable Haemophilus influenzae. J. Immunol. 2016, 196, 2742–2752. [Google Scholar] [CrossRef] [Green Version]
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell 2013, 153, 112–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramon, S.; Baker, S.F.; Sahler, J.M.; Kim, N.; Feldsott, E.A.; Serhan, C.N.; Martínez-Sobrido, L.; Topham, D.J.; Phipps, R.P. The specialized proresolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: A new class of adjuvant? J. Immunol. 2014, 193, 6031–6040. [Google Scholar] [CrossRef] [Green Version]
- Berrington de Gonzalez, A.; Hartge, P.; Cerhan, J.R.; Flint, A.J.; Hannan, L.; MacInnis, R.J.; Moore, S.C.; Tobias, G.S.; Anton-Culver, H.; Freeman, L.B.; et al. Body-mass index and mortality among 1.46 million white adults. N. Engl. J. Med. 2010, 363, 2211–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, K.J.; Nati, M.; Chavakis, T.; Chatzigeorgiou, A. Innate immune cells in the adipose tissue. Rev. Endocr. Metab. Disord. 2018, 19, 283–292. [Google Scholar] [CrossRef]
- Sugimoto, S.; Mena, H.A.; Sansbury, B.E.; Kobayashi, S.; Tsuji, T.; Wang, C.-H.; Yin, X.; Huang, T.L.; Kusuyama, J.; Kodani, S.D.; et al. Brown adipose tissue-derived MaR2 contributes to cold-induced resolution of inflammation. Nat. Metab. 2022, 4, 775–790. [Google Scholar] [CrossRef]
- Titos, E.; Rius, B.; López-Vicario, C.; Alcaraz-Quiles, J.; García-Alonso, V.; Lopategi, A.; Dalli, J.; Lozano, J.J.; Arroyo, V.; Delgado, S.; et al. Signaling and immunoresolving actions of resolvin D1 in inflamed human visceral adipose tissue. J. Immunol. 2016, 197, 3360–3370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titos, E.; Rius, B.; González-Périz, A.; López-Vicario, C.; Morán-Salvador, E.; Martínez-Clemente, M.; Arroyo, V.; Clària, J. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J. Immunol. 2011, 187, 5408–5418. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Fernández, L.; González-Muniesa, P.; Laiglesia, L.M.; Sáinz, N.; Prieto-Hontoria, P.L.; Escoté, X.; Odriozola, L.; Corrales, F.J.; Arbones-Mainar, J.M.; Martínez, J.A.; et al. Maresin 1 improves insulin sensitivity and attenuates adipose tissue inflammation in ob/ob and diet-induced obese mice. FASEB J. 2017, 31, 2135–2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clária, J.; Dalli, J.; Yacoubian, S.; Gao, F.; Serhan, C.N. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat1. J. Immunol. 2012, 189, 2597–2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuhofer, A.; Zeyda, M.; Mascher, D.; Itariu, B.K.; Murano, I.; Leitner, L.; Hochbrugger, E.E.; Fraisl, P.; Cinti, S.; Serhan, C.N.; et al. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes 2013, 62, 1945–1956. [Google Scholar] [CrossRef] [Green Version]
- White, P.J.; Arita, M.; Taguchi, R.; Kang, J.X.; Marette, A. Transgenic restoration of long-chain n-3 fatty acids in insulin target tissues improves resolution capacity and alleviates obesity-linked inflammation and insulin resistance in high-fat-fed mice. Diabetes 2010, 59, 3066–3073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellmann, J.; Tang, Y.; Kosuri, M.; Bhatnagar, A.; Spite, M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 2011, 25, 2399–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Fernández, L.; González-Muniesa, P.; Sáinz, N.; Escoté, X.; Martínez, J.A.; Arbones-Mainar, J.M.; Moreno-Aliaga, M.J. Maresin 1 regulates insulin signaling in human adipocytes as well as in adipose tissue and muscle of lean and obese mice. J. Physiol. Biochem. 2021, 77, 167–173. [Google Scholar] [CrossRef]
- Martínez-Fernández, L.; González-Muniesa, P.; Sáinz, N.; Laiglesia, L.M.; Escoté, X.; Martínez, J.A.; Moreno-Aliaga, M.J. Maresin 1 regulates hepatic FGF21 in diet-induced obese mice and in cultured hepatocytes. Mol. Nutr. Food Res. 2019, 63, e1900358. [Google Scholar] [CrossRef]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef] [Green Version]
- Bäck, M.; Yurdagul, A.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Fredman, G.; Hellmann, J.; Proto, J.D.; Kuriakose, G.; Colas, R.A.; Dorweiler, B.; Connolly, E.S.; Solomon, R.; Jones, D.M.; Heyer, E.J.; et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat. Commun. 2016, 7, 12859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viola, J.R.; Lemnitzer, P.; Jansen, Y.; Csaba, G.; Winter, C.; Neideck, C.; Silvestre-Roig, C.; Dittmar, G.; Döring, Y.; Drechsler, M.; et al. Resolving lipid mediators maresin 1 and resolvin D2 prevent atheroprogression in mice. Circ. Res. 2016, 119, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Lopategi, A.; Flores-Costa, R.; Rius, B.; López-Vicario, C.; Alcaraz-Quiles, J.; Titos, E.; Clària, J. Frontline Science: Specialized proresolving lipid mediators inhibit the priming and activation of the macrophage NLRP3 inflammasome. J. Leukoc. Biol. 2019, 105, 25–36. [Google Scholar] [CrossRef] [Green Version]
- D′elia, R.V.; Harrison, K.; Oyston, P.C.; Lukaszewski, R.A.; Clark, G.C. Targeting the “cytokine storm” for therapeutic benefit. Clin. Vaccine Immunol. 2013, 20, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.; Sharma, A.; Chen, M.; Toy, R.; Mottola, G.; Conte, M.S. The pro-resolving lipid mediator maresin 1 (MaR1) attenuates inflammatory signaling pathways in vascular smooth muscle and endothelial cells. PLoS ONE 2014, 9, e113480. [Google Scholar] [CrossRef]
- Norling, L.V.; Dalli, J.; Flower, R.J.; Serhan, C.N.; Perretti, M. Resolvin D1 limits PMN recruitment to inflammatory loci: Receptor dependent bioactions. Arter. Thromb. Vasc. Biol. 2012, 32, 1970–1978. [Google Scholar] [CrossRef] [Green Version]
- Önal, M.A.; Fentoğlu, Ö.; Aksoy, F.; Calapoğlu, M.; Varol, E.; Orhan, H. Salivary levels of last generation specific pro-resolving lipid mediators (SPMs) (protectin and maresin) in patients with cardiovascular and periodontal disease: A case-control study. J. Periodontal. Res. 2021, 56, 606–615. [Google Scholar] [CrossRef]
- Wang, H.B.; Yang, J.; Ding, J.W.; Chen, L.H.; Li, S.; Liu, X.W.; Yang, C.J.; Fan, Z.X.; Yang, J. RNAi-mediated down-regulation of CD47 protects against ischemia/reperfusion-induced myocardial damage via activation of enos in a rat model. Cell Physiol. Biochem. 2016, 40, 1163–1174. [Google Scholar] [CrossRef]
- Gilbert, K.; Bernier, J.; Bourque-Riel, V.; Malick, M.; Rousseau, G. Resolvin D1 reduces infarct size through a phosphoinositide 3-kinase/protein kinase B mechanism. J. Cardiovasc. Pharmacol. 2015, 66, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Kain, V.; Ingle, K.A.; Colas, R.A.; Dalli, J.; Prabhu, S.D.; Serhan, C.N.; Joshi, M.D.; Halade, G.V. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J. Mol. Cell. Cardiol. 2015, 84, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Pope, N.H.; Salmon, M.; Davis, J.P.; Chatterjee, A.; Su, G.; Conte, M.S.; Ailawadi, G.; Upchurch, G.R. D-series resolvins inhibit murine abdominal aortic aneurysm formation and increase M2 macrophage polarization. FASEB J. 2016, 30, 4192–4201. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, K.; Bernier, J.; Godbout, R.; Rousseau, G. Resolvin D1, a metabolite of omega-3 polyunsaturated fatty acid, decreases post-myocardial infarct depression. Mar. Drugs 2014, 12, 5396–5407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulnour, R.E.; Dalli, J.; Colby, J.K.; Krishnamoorthy, N.; Timmons, J.Y.; Tan, S.H.; Colas, R.A.; Petasis, N.A.; Serhan, C.N.; Levy, B.D. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc. Natl. Acad. Sci. USA 2014, 111, 16526–16531. [Google Scholar] [CrossRef]
- Lannan, K.L.; Spinelli, S.L.; Blumberg, N.; Phipps, R.P. Maresin 1 induces a novel pro-resolving phenotype in human platelets. J. Thromb. Haemost. 2017, 15, 802–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Hernández, C.D.; Torres-Alarcón, L.A.; González-Cortés, A.; Peón, A.N.; Rungatscher, A. Ischemia/reperfusion injury: Pathophysiology, current clinical management, and potential preventive approaches. Mediat. Inflamm. 2020, 2020, 8405370. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, H.; Jin, H.; Liu, K. Role of inflammatory factors in mediating the effect of lipids on nonalcoholic fatty liver disease: A two-step, multivariable mendelian randomization study. Nutrients 2022, 14, 4434. [Google Scholar] [CrossRef]
- Herrera Vielma, F.; Valenzuela, R.; Videla, L.A.; Zúñiga-Hernández, J. N-3 polyunsaturated fatty acids and their lipid mediators as a potential immune–nutritional intervention: A molecular and clinical view in hepatic disease and other non-communicable illnesses. Nutrients 2021, 13, 3384. [Google Scholar] [CrossRef]
- Laiglesia, L.M.; Lorente-Cebrián, S.; Martínez-Fernández, L.; Sáinz, N.; Prieto-Hontoria, P.L.; Burrell, M.Á.; Rodriguez-Ortigosa, C.M.; Martínez, J.A.; Moreno-Aliaga, M.J. Maresin 1 mitigates liver steatosis in ob/ob and diet-induced obese mice. Int. J. Obes. 2018, 42, 572–579. [Google Scholar] [CrossRef]
- Jung, T.W.; Kim, H.C.; El-Aty, A.M.A.; Jeong, J.H. Maresin 1 attenuates NAFLD by suppression of endoplasmic reticulum stress via AMPK-SERCA2b pathway. J. Biol. Chem. 2018, 293, 3981–3988. [Google Scholar] [CrossRef] [Green Version]
- Rius, B.; Duran-Güell, M.; Flores-Costa, R.; López-Vicario, C.; Lopategi, A.; Alcaraz-Quiles, J.; Casulleras, M.; Jose Lozano, J.; Titos, E.; Claria, J. The specialized proresolving lipid mediator maresin 1 protects hepatocytes from lipotoxic and hypoxia-induced endoplasmic reticulum stress. FASEB J. 2017, 31, 5384–5398. [Google Scholar] [CrossRef] [Green Version]
- Jung, T.W.; Hwang, H.J.; Hong, H.C.; Choi, H.Y.; Yoo, H.J.; Baik, S.H.; Choi, K.M. Resolvin D1 reduces ER stress-induced apoptosis and triglyceride accumulation through JNK pathway in HepG2 cells. Mol. Cell Endocrinol. 2014, 39, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.W.; Kyung, E.J.; Kim, H.C.; Shin, Y.K.; Lee, S.H.; Park, E.S.; Hacımüftüoğlu, A.; El-Aty, A.M.A.; Jeong, J.H. Protectin DX ameliorates hepatic steatosis by suppression of endoplasmic reticulum stress via AMPK-Induced ORP150 expression. J. Pharmacol. Exp. Ther. 2018, 365, 485–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.-H.; Shin, K.-O.; Kim, J.-Y.; Khadka, D.B.; Kim, H.-J.; Lee, Y.-M.; Cho, W.-J.; Cha, J.-Y.; Lee, B.-J.; Lee, M.-O. A maresin 1/RORα/12-lipoxygenase autoregulatory circuit prevents inflammation and progression of nonalcoholic steatohepatitis. J. Clin. Investig. 2019, 129, 1684–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rius, B.; Titos, E.; Arroyo, V.; Claria, J.; Morán-Salvador, E.; López-Vicario, C.; García-Alonso, V.; González-Périz, A. Resolvin D1 primes the resolution process initiated by calorie restriction in obesity-induced steatohepatitis. FASEB J. 2014, 28, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, H.; Ye, T.; Fu, X.; Tan, X.; Zeng, Y.; Fan, J.; Xu, Y. Low serum Maresin-1 levels are associated with non-alcoholic fatty liver disease: A cross-sectional study. Lipids Health Dis. 2021, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.J.; Sabaj, M.; Tolosa, G.; Herrera Vielma, F.; Zúñiga, M.J.; González, D.R.; Zúñiga-Hernández, J. Maresin-1 prevents liver fibrosis by targeting Nrf2 and NF-κB, reducing oxidative stress and inflammation. Cells 2021, 10, 3406. [Google Scholar] [CrossRef]
- Valenzuela, R.; Videla, L.A. Impact of the co-administration of n-3 fatty acids and olive oil components in preclinical nonalcoholic fatty liver disease models: A mechanistic view. Nutrients 2020, 12, 499. [Google Scholar] [CrossRef] [Green Version]
- Echeverría, F.; Valenzuela, R.; Espinosa, A.; Bustamante, A.; Álvarez, D.; Gonzalez-Mañan, D.; Ortiz, M.; Soto-Alarcon, S.A.; Videla, L.A. Reduction of high-fat diet-induced liver proinflammatry state by eicosapentaenoic acid plus hydroxytyrosol supplementation: Involvement of resolvins RvE1/2 and RvD1/2. J. Nutr. Biochem. 2019, 63, 35–43. [Google Scholar] [CrossRef]
- Soto-Alarcón, S.A.; Ortiz, M.; Orellana, P.; Echeverría, F.; Gonzalez-Mañán, D.; Bustamante, A.; Espinosa, A.; Illesca, P.; Valenzuela, R.; Videla, L.A. Docosahexaenoic acid and hydroxytyrosol co-administration fully prevent liver steatosis and related parameters in mice subjected to high-fat diet: A molecular approach. BioFactors 2019, 45, 930–943. [Google Scholar] [CrossRef] [PubMed]
- D’Espessailles, A.; Dossi, C.; Intriago, G.; Leiva, P.; Romanque, P. Hormonal pretreatment preserves liver regenerative capacity and minimizes inflammation after partial hepatectomy. Ann. Hepatol. 2013, 12, 881–891. [Google Scholar] [CrossRef]
- Mardones, M.; Valenzuela, R.; Romanque, P.; Covarrubias, N.; Anghileri, F.; Fernández, V.; Videla, L.A.; Tapia, G. Prevention of liver ischemia reperfusion injury by a combined thyroid hormone and fish oil protocol. J. Nutr. Biochem. 2012, 23, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Dartt, D.A.; Masli, S. Conjunctival epithelial and goblet cell function in chronic inflammation and ocular allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Hodges, R.R.; Dartt, D.A. Tear film mucins: Front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucins. Exp. Eye Res. 2013, 117, 62–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, M.V.; Lyngstadaas, A.V.; Bair, J.A.; Hodges, R.R.; Utheim, T.P.; Serhan, C.N.; Dartt, D.A. Signaling pathways used by the specialized pro-resolving mediator maresin 2 regulate goblet cell function: Comparison with maresin 1. Int. J. Mol. Sci. 2022, 23, 6233. [Google Scholar] [CrossRef] [PubMed]
- Kaye, R.; Botten, N.; Lippestad, M.; Li, D.; Hodges, R.R.; Utheim, T.P.; Serhan, C.N.; Dartt, D.A. Resolvin D1, but not resolvin E1, transactivates the epidermal growth factor receptor to increase intracellular calcium and glycoconjugate secretion in rat and human conjunctival goblet cells. Exp. Eye Res. 2019, 180, 53–62. [Google Scholar] [CrossRef]
- Trotta, M.C.; Gharbia, S.; Herman, H.; Mladin, B.; Hermenean, A.; Balta, C.; Cotoraci, C.; Peteu, V.E.; Gesualdo, C.; Petrillo, F.; et al. Sex and age-related differences in neuroinflammation and apoptosis in balb/c mice retina involve resolvin D1. Int. J. Mol. Sci. 2021, 22, 6280. [Google Scholar] [CrossRef]
- Ferguson, B.; Bokka, N.R.; Maddipati, K.R.; Ayilavarapu, S.; Weltman, R.; Zhu, L.; Chen, W.; Zheng, W.J.; Angelov, N.; Van Dyke, T.E.; et al. Distinct profiles of specialized pro-resolving lipid mediators and corresponding receptor gene expression in periodontal inflammation. Front. Immunol. 2020, 11, 1307. [Google Scholar] [CrossRef]
- Alikhani, M.; Alyami, B.; Lee, I.S.; Almoammar, S.; Vongthongleur, T.; Alikhani, M.; Alansari, S.; Sangsuwon, C.; Chou, M.Y.; Khoo, E.; et al. Saturation of the biological response to orthodontic forces and its effect on the rate of tooth movement. Orthod. Craniofacial Res. 2015, 18, 8–17. [Google Scholar] [CrossRef]
- Klein, Y.; Levin-Talmor, O.; Berkstein, J.G.; Wald, S.; Meirow, Y.; Maimon, A.; Leibovich, A.; Barenholz, Y.; Polak, D.; Chaushu, S. Resolvin D1 shows osseous-protection via RANK reduction on monocytes during orthodontic tooth movement. Front. Immunol. 2022, 13, 928132. [Google Scholar] [CrossRef]
- Park, K.D.; Kim, N.; Kang, J.; Dhakal, H.; Kim, J.Y.; Jang, Y.H.; Lee, W.J.; Lee, S.J.; Kim, S.H. Protectin D1 reduces imiquimod-induced psoriasiform skin inflammation. Int. Immunopharmacol. 2021, 98, 107883. [Google Scholar] [CrossRef]
- Signorini, C.; Moretti, E.; Noto, D.; Micheli, L.; Ponchia, R.; Collodel, G. Fatty acid oxidation and pro-resolving lipid mediators are related to male infertility. Antioxidants 2022, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Arnardottir, H.H.; Dalli, J.; Norling, L.V.; Colas, R.A.; Perretti, M.; Serhan, C.N. Resolvin D3 is dysregulated in arthritis and reduces arthritic inflammation. J. Immunol. 2016, 197, 2362–2368. [Google Scholar] [CrossRef] [Green Version]
- Hughes, F.M.; Allkanjari, A.; Odom, M.R.; Jin, H.; Purves, J.T. Specialized pro-resolution mediators in the bladder: Receptor expression and recovery of bladder function from cystitis. Exp. Biol. Med. 2022, 247, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, X.Y.; Fang, X.; Teng, F.Y.; Xu, Y. Decreased serum maresin 1 concentration is associated with postmenopausal osteoporosis: A cross-sectional study. Front. Med. 2022, 8, 759825. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyer, M.P.; Videla, L.A.; Farías, C.; Valenzuela, R. Potential Clinical Applications of Pro-Resolving Lipids Mediators from Docosahexaenoic Acid. Nutrients 2023, 15, 3317. https://doi.org/10.3390/nu15153317
Beyer MP, Videla LA, Farías C, Valenzuela R. Potential Clinical Applications of Pro-Resolving Lipids Mediators from Docosahexaenoic Acid. Nutrients. 2023; 15(15):3317. https://doi.org/10.3390/nu15153317
Chicago/Turabian StyleBeyer, María Paz, Luis A. Videla, Camila Farías, and Rodrigo Valenzuela. 2023. "Potential Clinical Applications of Pro-Resolving Lipids Mediators from Docosahexaenoic Acid" Nutrients 15, no. 15: 3317. https://doi.org/10.3390/nu15153317







