Gut Microbiome Is Related to Cognitive Impairment in Peritoneal Dialysis Patients
Highlights
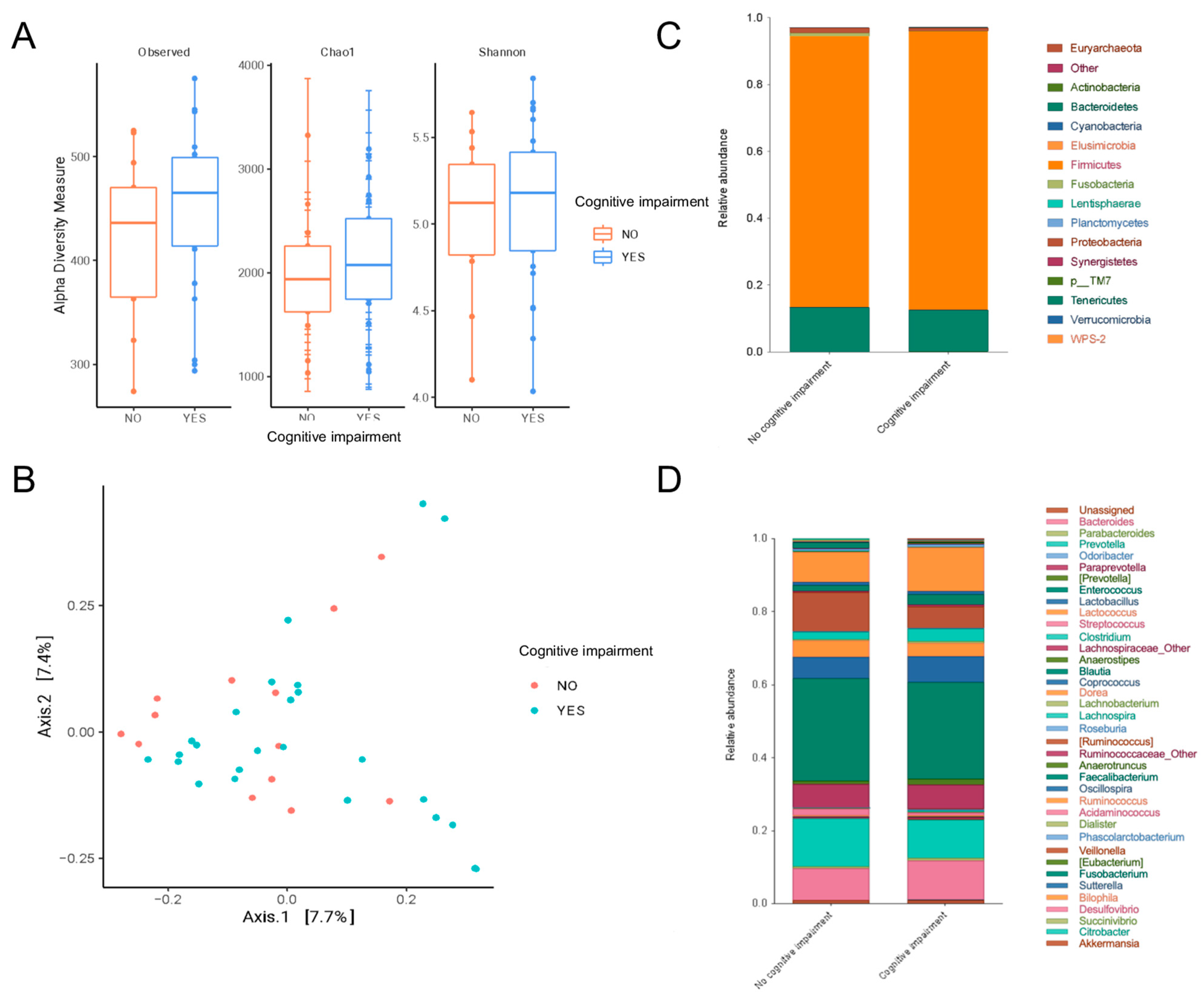
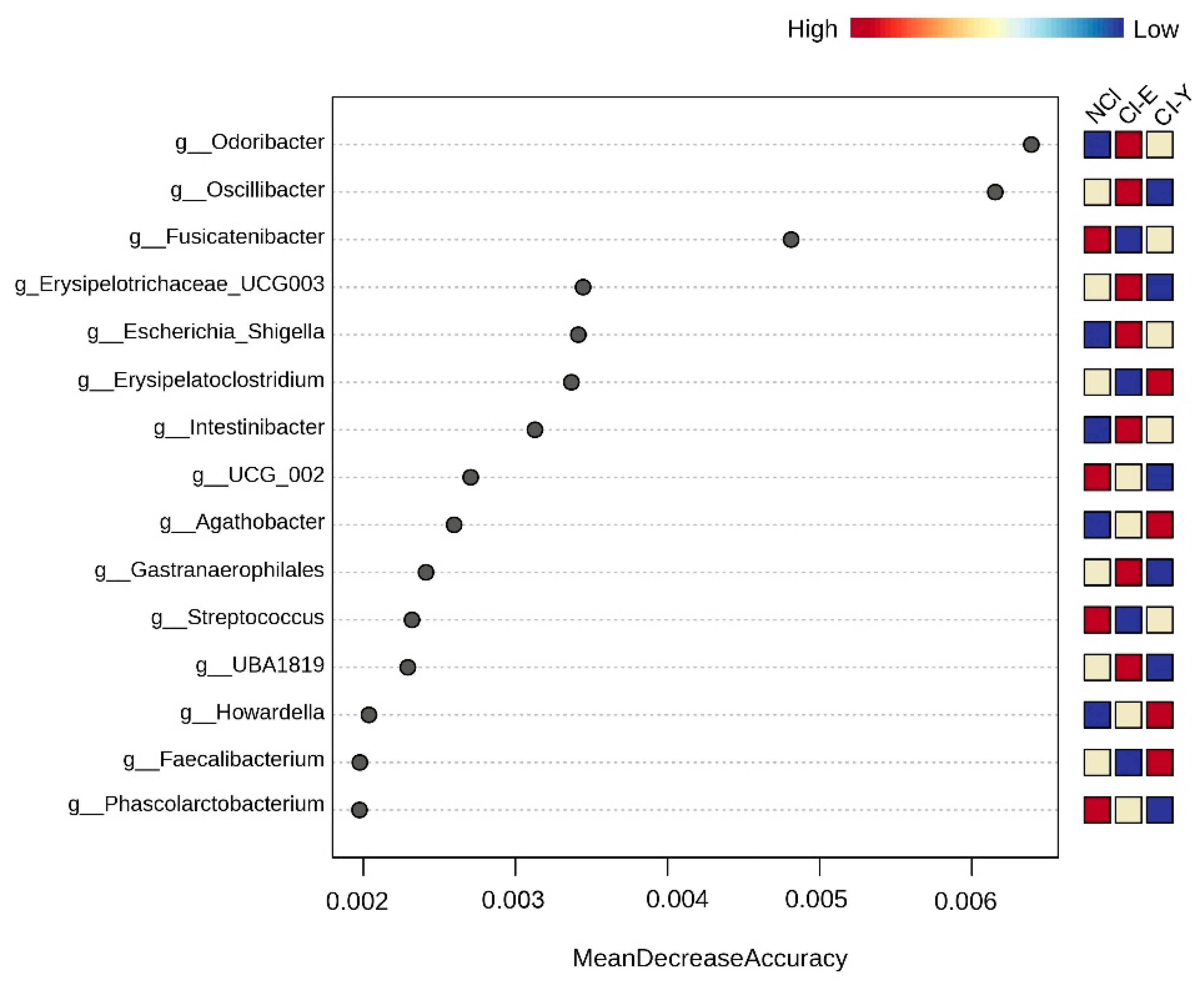
- Peritoneal dialysis patients with mild cognitive impairment had a gut microbiota enriched in Odoribacter, Anaerotruncus, S24_7 and Rikenellaceae.
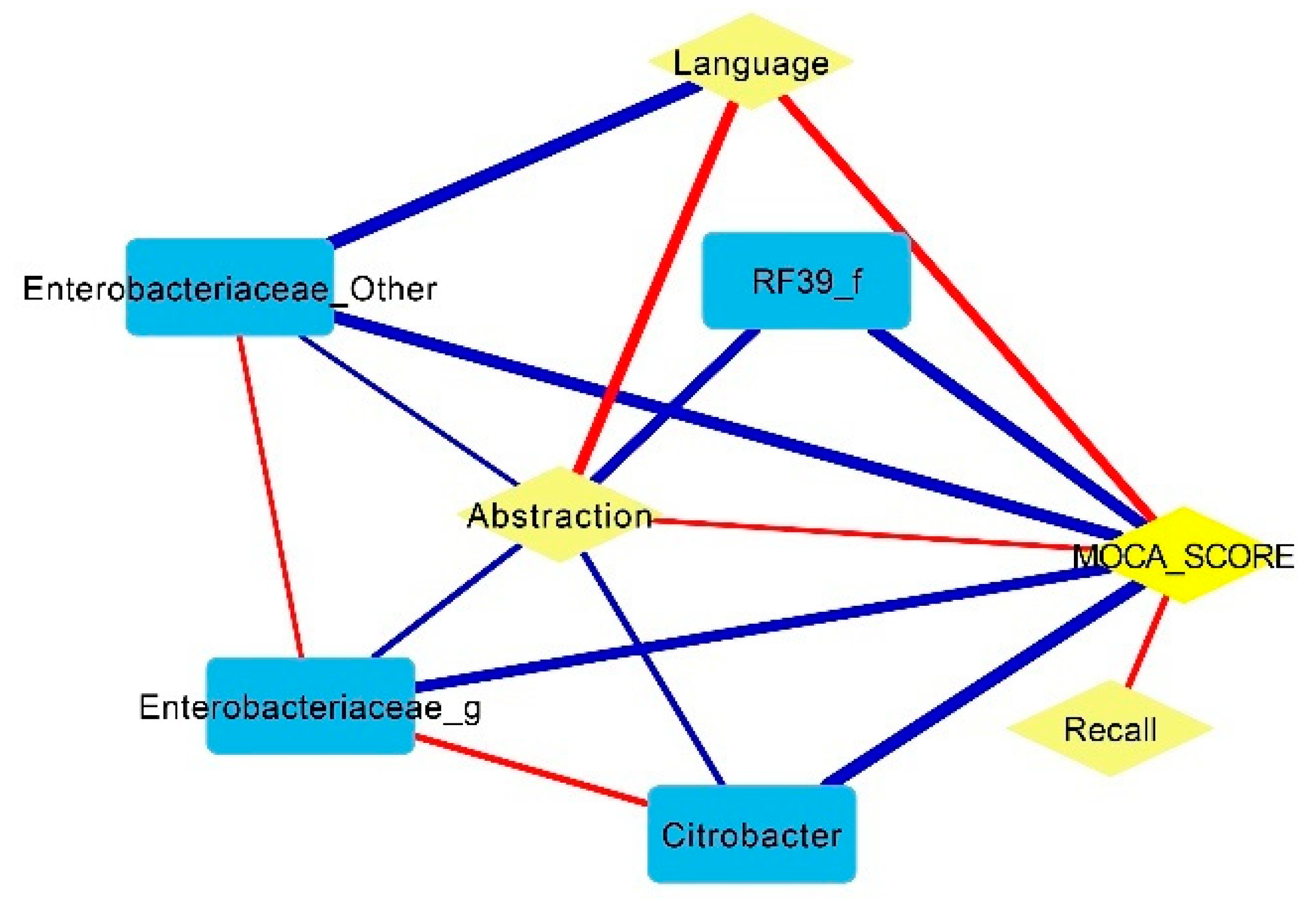
- Some pathobionts such as Enterobacteriaceae and Citrobacter had a negative correlation with cognitive function (abstraction and language of MoCA scores), while some short-chain-fatty-acid-producing bacteria such as Prevotella and Bifidobacterium were positively associated with cognitive function.
- Mucolytic bacteria such as Odoribacter, Intestinibacter and UBA1819 were associated with cognitive impairment after adjustments based on glucose levels and age.
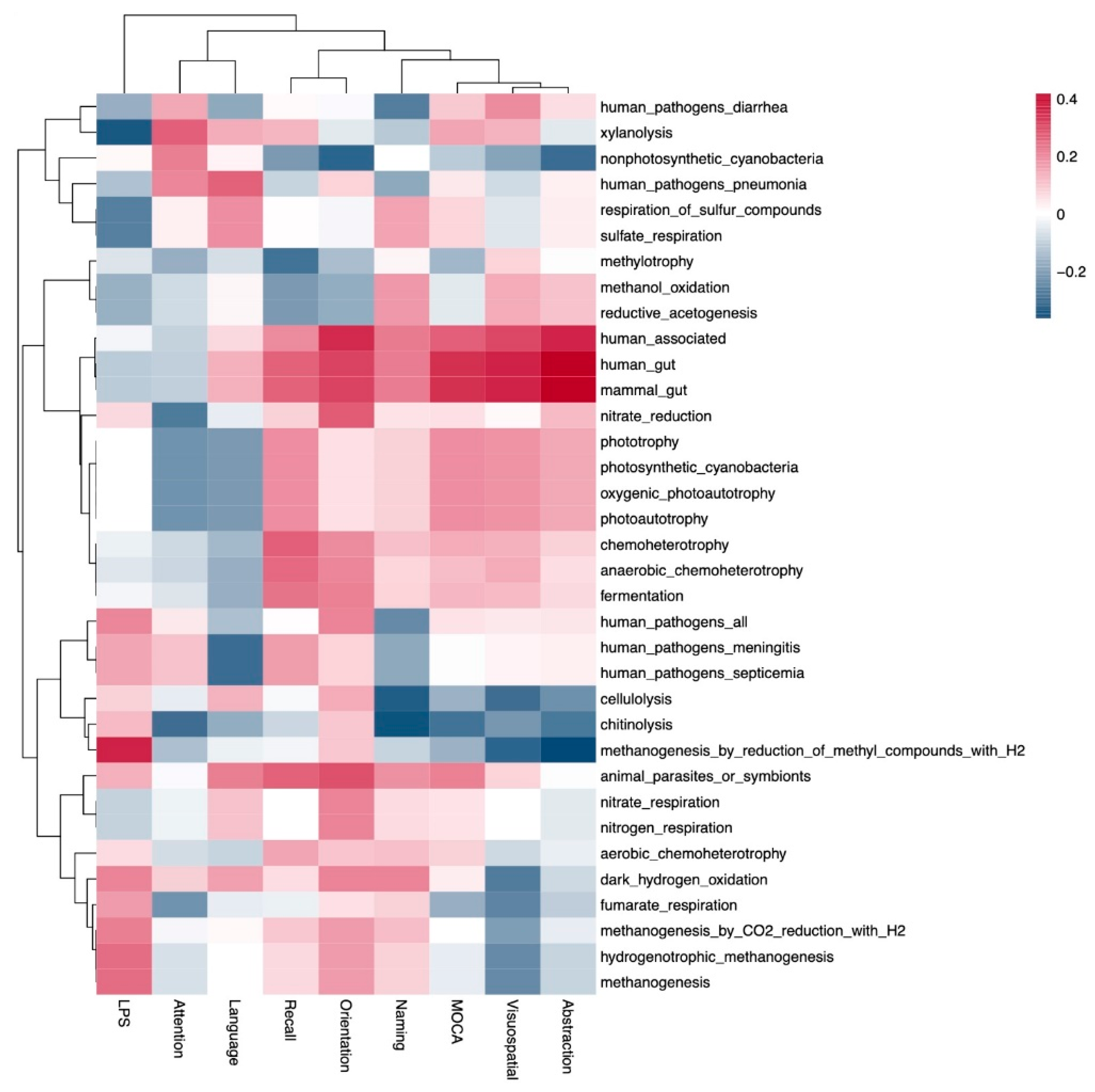
- This study shows evidence linking gut metabolism with cognitive function in patients on peritoneal dialysis.
- Further studies on dietary interventions aimed at modifying the gut microbiota are needed to evaluate their impact on cognition in peritoneal dialysis patients.
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berger, I.; Wu, S.; Masson, P.; Kelly, P.J.; Duthie, F.A.; Whiteley, W.; Parker, D.; Gillespie, D.; Webster, A.C. Cognition in chronic kidney disease: A systematic review and meta-analysis. BMC Med. 2016, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; An, R.; Wang, Y.; Lei, J.; Liang, J.; Wan, Q. Risk factors and prevalence of cognitive impairment in maintenance haemodialysis patients: A systematic review and meta-analysis of observational studies. J. Adv. Nurs. 2023, 79, 3691–3706. [Google Scholar] [CrossRef] [PubMed]
- Shea, Y.F.; Lee, M.C.; Mok, M.M.; Chan, F.H.; Chan, T.M. Prevalence of cognitive impairment among peritoneal dialysis patients: A systematic review and meta-analysis. Clin. Exp. Nephrol. 2019, 23, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Félix, N.A.; Martin-del-Campo, F.; Cueto-Manzano, A.M.; Romo-Flores, M.L.; Velázquez-Vidaurri, A.L.; Sánchez-Soriano, A.; Ruvalcaba-Contreras, N.; Calderón-Fabian, A.; Rojas-Campos, E.; Cortés-Sanabria, L. Prevalence of mild cognitive impairment in automated peritoneal dialysis patients. Nephrol. Dial. Transpl. 2021, 36, 2106–2111. [Google Scholar] [CrossRef] [PubMed]
- Shea, Y.F.; Lee, M.S.; Mok, M.Y.; Lam, M.F.; Chu, L.W.; Chan, F.H.; Chan, T.M. Self-Care Peritoneal Dialysis Patients with Cognitive Impairment Have a Higher Risk of Peritonitis in the Second Year. Perit. Dial. Int. 2019, 39, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yi, C.; Wu, M.; Qiu, Y.; Wu, H.; Ye, H.; Peng, Y.; Xiao, X.; Lin, J.; Yu, X.; et al. Risk Factors and Clinical Outcomes of Cognitive Impairment in Diabetic Patients Undergoing Peritoneal Dialysis. Kidney Blood Press. Res. 2021, 46, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Tong, S.; Chu, X.; Feng, T.; Geng, M. Chronic Kidney Disease and Cognitive Impairment: The Kidney-Brain Axis. Kidney Dis. 2022, 8, 275–285. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, C. The Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders: Pathophysiological Mechanisms and Novel Treatments. Curr. Neuropharmacol. 2018, 16, 559–573. [Google Scholar] [CrossRef]
- Lun, H.; Yang, W.; Zhao, S.; Jiang, M.; Xu, M.; Liu, F.; Wang, Y. Altered gut microbiota and microbial biomarkers associated with chronic kidney disease. Microbiologyopen 2019, 8, e00678. [Google Scholar] [CrossRef]
- Wang, X.; Yang, S.; Li, S.; Zhao, L.; Hao, Y.; Qin, J.; Zhang, L.; Zhang, C.; Bian, W.; Zuo, L.I.; et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 2020, 69, 2131–2142. [Google Scholar] [CrossRef]
- Meijers, B.; Evenepoel, P.; Anders, H.J. Intestinal microbiome and fitness in kidney disease. Nat. Rev. Nephrol. 2019, 15, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, Y.; Shen, Y.; Zhang, Y.; Liu, L.; Yang, X. Dietary polyphenols: Regulate the advanced glycation end products-RAGE axis and the microbiota-gut-brain axis to prevent neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2023, 63, 9816–9842. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhao, W.; Lin, Z.; Wu, J.; Lin, H.; Li, Y.; Song, J.; Zhang, J.; Peng, H. The Effects of Hemodialysis and Peritoneal Dialysis on the Gut Microbiota of End-Stage Renal Disease Patients, and the Relationship Between Gut Microbiota and Patient Prognoses. Front. Cell. Infect. Microbiol. 2021, 11, 579386. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Shen, J.; Jiang, R.; Jin, L.; Zhan, G.; Liu, J.; Sha, Q.; Xu, R.; Miao, L.; Yang, C. Abnormalities in gut microbiota and serum metabolites in hemodialysis patients with mild cognitive decline: A single-center observational study. Psychopharmacology 2020, 237, 2739–2752. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Zheng, L.J.; Liu, Y.; Ye, Y.B.; Luo, S.; Lu, G.M.; Gong, D.; Zhang, L.J. The gut microbiota-inflammation-brain axis in end-stage renal disease: Perspectives from default mode network. Theranostics 2019, 9, 8171–8181. [Google Scholar] [CrossRef]
- Yang, Q.; Li, R.; Zhong, Z.; Mao, H.; Fan, J.; Lin, J.; Yang, X.; Wang, X.; Li, Z.; Yu, X. Is cystatin C a better marker than creatinine for evaluating residual renal function in patients on continuous ambulatory peritoneal dialysis? Nephrol. Dial. Transpl. 2011, 26, 3358–3365. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation, Inc. NKF-DOQI Clinical Practice Guidelines for Peritoneal Dialysis Adequacy. Am. J. Kidney Dis. 1997, 30 (Suppl. S2), S67–S136. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhang, C.; Feng, H.; Yuan, J.; Ding, L.; Fang, W.; Gu, A.; Huang, J.; Li, N.; Gu, L.; et al. Clinical characteristics associated with the properties of gut microbiota in peritoneal dialysis patients. Perit. Dial. Int. 2021, 41, 298–306. [Google Scholar] [CrossRef]
- Merino-Ribas, A.; Araujo, R.; Pereira, L.; Campos, J.; Barreiros, L.; Segundo, M.A.; Silva, N.; Costa, C.F.; Quelhas-Santos, J.; Trindade, F.; et al. Vascular Calcification and the Gut and Blood Microbiome in Chronic Kidney Disease Patients on Peritoneal Dialysis: A Pilot Study. Biomolecules 2022, 12, 867. [Google Scholar] [CrossRef]
- Gao, Q.; Li, D.; Wang, Y.; Zhao, C.; Li, M.; Xiao, J.; Kang, Y.; Lin, H.; Wang, N. Analysis of intestinal flora and cognitive function in maintenance hemodialysis patients using combined 16S ribosome DNA and shotgun metagenome sequencing. Aging Clin. Exp. Res. 2024, 36, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, S.; Zhang, J.; Li, Y.; Wu, Y.; Qi, X. Correlation between gut microbiome and cognitive impairment in patients undergoing peritoneal dialysis. BMC Nephrol. 2023, 24, 360. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xing, H.; Lin, W.; Yu, H.; Yang, B.; Jiang, C.; Zhang, J.; Wu, R.; Ding, F.; Pei, M.; et al. Specific gut microbiome and metabolome changes in patients with continuous ambulatory peritoneal dialysis and comparison between patients with different dialysis vintages. Front. Med. 2024, 10, 1302352. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Wang, N.; Han, M.; Ban, M.; Sun, T.; Xu, J. Reviewing the role of gut microbiota in the pathogenesis of depression and exploring new therapeutic options. Front. Neurosci. 2022, 16, 1029495. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Chang, C.C.; Huang, C.W.; Nouchi, R.; Cheng, C.H. Gut microbiota in patients with Alzheimer’s disease spectrum: A systematic review and meta-analysis. Aging 2022, 14, 477–496. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Ho Do, M.; Seo, Y.S.; Park, H.Y. Polysaccharides: Bowel health and gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Togo, A.H.; Diop, A.; Dubourg, G.; Khelaifia, S.; Richez, M.; Armstrong, N.; Maraninchi, M.; Fournier, P.E.; Raoult, D.; Million, M. Anaerotruncus massiliensis sp. nov., a succinate-producing bacterium isolated from human stool from an obese patient after bariatric surgery. New Microbes New Infect. 2019, 29, 100508. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Gao, Y.; Qiu, Y.; Jiang, S.; Zhang, Q.; Zhang, J.; Wang, L.; Zhang, Y.; Wang, L.; Nie, K. Gut Microbiota Altered in Mild Cognitive Impairment Compared With Normal Cognition in Sporadic Parkinson’s Disease. Front. Neurol. 2020, 11, 137. [Google Scholar] [CrossRef]
- Li, Z.; Liang, H.; Hu, Y.; Lu, L.; Zheng, C.; Fan, Y.; Wu, B.; Zou, T.; Luo, X.; Zhang, X.; et al. Gut bacterial profiles in Parkinson’s disease: A systematic review. CNS Neurosci. Ther. 2023, 29, 140–157. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.D.; Xiao, Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav. Immun. 2018, 70, 194–202. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Döring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B.; Wilmes, P. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 2018, 33, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Mo, X.; Huang, H.; Chen, X.; Liu, H.; Peng, Z.; Chen, L.; Rong, S.; Yang, W.; Xu, S.; et al. Yeast β-glucan alleviates cognitive deficit by regulating gut microbiota and metabolites in Aβ1-42-induced AD-like mice. Int. J. Biol. Macromol. 2020, 161, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Gao, M.; Liu, Z.; Zhang, Y.; Tu, H.; Lei, L.; Wu, P.; Zhang, A.; Yang, C.; Li, G.; et al. Gut Microbiome Composition Linked to Inflammatory Factors and Cognitive Functions in First-Episode, Drug-Naive Major Depressive Disorder Patients. Front. Neurosci. 2022, 15, 800764. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Ida, M.; Peterson, V.L.; Prenderville, J.A.; Moloney, G.M.; Izumo, T.; Murphy, K.; Murphy, A.; Ross, R.P.; Stanton, C.; et al. Revisiting Metchnikoff: Age-related alterations in microbiota-gut-brain axis in the mouse. Brain Behav. Immun. 2017, 65, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, L.; Ji, H.F. Alzheimer’s Disease Histological and Behavioral Manifestations in Transgenic Mice Correlate with Specific Gut Microbiome State. J. Alzheimer’s Dis. 2017, 56, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Hang, Z.; Cai, S.; Lei, T.; Zhang, X.; Xiao, Z.; Wang, D.; Li, Y.; Bi, W.; Yang, Y.; Deng, S.; et al. Transfer of Tumor-Bearing Mice Intestinal Flora Can Ameliorate Cognition in Alzheimer’s Disease Mice. J. Alzheimer’s Dis. 2022, 86, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Fu, Y.; Cao, W.T.; Wang, Z.; Zhang, K.; Jiang, Z.; Jia, X.; Liu, C.Y.; Lin, H.R.; Zhong, H.; et al. Gut microbiome, cognitive function and brain structure: A multi-omics integration analysis. Transl. Neurodegener. 2022, 11, 49. [Google Scholar] [CrossRef]
- Brandsma, E.; Kloosterhuis, N.J.; Koster, M.; Dekker, D.C.; Gijbels, M.J.; Van Der Velden, S.; Ríos-Morales, M.; Van Faassen, M.J.; Loreti, M.G.; De Bruin, A.; et al. A Proinflammatory Gut Microbiota Increases Systemic Inflammation and Accelerates Atherosclerosis. Circ. Res. 2019, 124, 94–100. [Google Scholar] [CrossRef]
- Nagai, F.; Morotomi, M.; Watanabe, Y.; Sakon, H.; Tanaka, R. Alistipes indistinctus sp. nov. and Odoribacter laneus sp. nov., common members of the human intestinal microbiota isolated from faeces. Int. J. Syst. Evol. Microbiol. 2010, 60 Pt 6, 1296–1302. [Google Scholar] [CrossRef]
- Lin, Y.T.; Wu, P.H.; Lee, H.H.; Mubanga, M.; Chen, C.S.; Kuo, M.C.; Chiu, Y.W.; Kuo, P.L.; Hwang, S.J. Indole-3 acid increased risk of impaired cognitive function in patients receiving hemodialysis. Neurotoxicology 2019, 73, 85–91. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Huang, M.F.; Liang, S.S.; Hwang, S.J.; Tsai, J.C.; Liu, T.L.; Wu, P.H.; Yang, Y.H.; Kuo, K.C.; Kuo, M.C.; et al. Indoxyl sulfate, not p-cresyl sulfate, is associated with cognitive impairment in early-stage chronic kidney disease. Neurotoxicology 2016, 53, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Bang, S.J.; Kim, G.; Lim, M.Y.; Song, E.J.; Jung, D.H.; Kum, J.S.; Nam, Y.D.; Park, C.S.; Seo, D.H. The influence of in vitro pectin fermentation on the human fecal microbiome. AMB Express 2018, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Qiu, W.; Zhu, X.; Li, X.; Xie, Z.; Carreras, I.; Dedeoglu, A.; Van Dyke, T.; Han, Y.W.; Karimbux, N.; et al. The Periodontal Pathogen Fusobacterium nucleatum Exacerbates Alzheimer’s Pathogenesis via Specific Pathways. Front. Aging Neurosci. 2022, 14, 912709. [Google Scholar] [CrossRef]
- Zhao, J.; Ning, X.; Liu, B.; Dong, R.; Bai, M.; Sun, S. Specific alterations in gut microbiota in patients with chronic kidney disease: An updated systematic review. Ren. Fail. 2021, 43, 102–112. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Zhao, Y.-Y.; Pahl, M.V. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: The nature, mechanisms, consequences and potential treatment. Nephrol. Dial. Transpl. 2016, 31, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Mulder, D.; Aarts, E.; Arias Vasquez, A.; Bloemendaal, M. A systematic review exploring the association between the human gut microbiota and brain connectivity in health and disease. Mol. Psychiatry 2023, 28, 5037–5061. [Google Scholar] [CrossRef]
- Cooke, M.B.; Catchlove, S.; Tooley, K.L. Examining the Influence of the Human Gut Microbiota on Cognition and Stress: A Systematic Review of the Literature. Nutrients 2022, 14, 4623. [Google Scholar] [CrossRef]
- Kohn, N.; Szopinska-Tokov, J.; Llera Arenas, A.; Beckmann, C.F.; Arias-Vasquez, A.; Aarts, E. Multivariate associative patterns between the gut microbiota and large-scale brain network connectivity. Gut Microbes 2021, 13, 2006586. [Google Scholar] [CrossRef] [PubMed]
- Eicher, T.P.; Mohajeri, M.H. Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases. Nutrients 2022, 14, 2661. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, A.; Bastiaanssen, T.F.; Cryan, J.F.; Tinahones, F.J.; Vioque, J.; Corella, D.; Fitó, M.; Vidal, J.; Moreno-Indias, I.; Gómez-Pérez, A.M.; et al. Taxonomic and Functional Fecal Microbiota Signatures Associated with Insulin Resistance in Non-Diabetic Subjects with Overweight/Obesity within the Frame of the PREDIMED-Plus Study. Front. Endocrinol. 2022, 13, 804455. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Lin, J.S.; Mao, Y.; Chen, G.D.; Zeng, F.F.; Dong, H.L.; Jiang, Z.; Wang, J.; Xiao, C.; Shuai, M.; et al. Erythrocyte n-6 Polyunsaturated Fatty Acids, Gut Microbiota, and Incident Type 2 Diabetes: A Prospective Cohort Study. Diabetes Care 2020, 43, 2435–2443. [Google Scholar] [CrossRef]
- Meynier, M.; Daugey, V.; Mallaret, G.; Gervason, S.; Meleine, M.; Barbier, J.; Aissouni, Y.; Lolignier, S.; Bonnet, M.; Ardid, D.; et al. Pasteurized akkermansia muciniphila improves irritable bowel syndrome-like symptoms and related behavioral disorders in mice. Gut Microbes 2024, 16, 2298026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, H.; Wang, Y.; Cheng, R.; Pu, F.; Yang, Y.; Li, J.; Wu, S.; Shen, X.; He, F. Heat-inactivated Lacticaseibacillus paracasei N1115 alleviates the damage due to brain function caused by long-term antibiotic cocktail exposure in mice. BMC Neurosci. 2022, 23, 38. [Google Scholar] [CrossRef]
- Chao, C.T.; Lee, S.Y.; Yang, W.S.; Chen, H.W.; Fang, C.C.; Yen, C.J.; Chiang, C.K.; Hung, K.Y.; Huang, J.W. Citrobacter peritoneal dialysis peritonitis: Rare occurrence with poor outcomes. Int. J. Med. Sci. 2013, 10, 1092–1098. [Google Scholar] [CrossRef]
- Labarthe, S.; Plancade, S.; Raguideau, S.; Plaza Oñate, F.; Le Chatelier, E.; Leclerc, M.; Laroche, B. Four functional profiles for fibre and mucin metabolism in the human gut microbiome. Microbiome 2023, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.Y.; Chen, Y.S.; Liu, Z. Relationship among Parkinson’s disease, constipation, microbes, and microbiological therapy. World J. Gastroenterol. 2024, 30, 225–237. [Google Scholar] [CrossRef]
- Campbell, A.; Gdanetz, K.; Schmidt, A.W.; Schmidt, T.M. H2 generated by fermentation in the human gut microbiome influences metabolism and competitive fitness of gut butyrate producers. Microbiome 2023, 11, 133. [Google Scholar] [CrossRef]

| Variable | Normal Cognitive Function (n = 14) | Mild Cognitive Impairment (n = 25) | p |
|---|---|---|---|
| Age (years) | 38 ± 14 | 53 ± 16 | 0.006 |
| Female sex, n (%) | 2 (14) | 5 (20) | 0.66 |
| Marital status, n (%) | 0.60 | ||
| Single | 5 (36) | 5 (20) | |
| Married | 7 (50) | 18 (72) | |
| Widowed/divorced | 2 (14) | 2 (8) | |
| Educational level, n (%) | 0.49 | ||
| Elementary/middle school | 7 (50) | 17 (68) | |
| High school/technical career | 5 (36) | 5 (20) | |
| Professional | 2 (14) | 3 (12) | |
| Diabetes mellitus, n (%) | 3 (21) | 14 (56) | 0.04 |
| Hypertension, n (%) | 11 (79) | 22 (88) | 0.65 |
| Cardiovascular disease, n (%) | 1 (7) | 3 (12) | 0.63 |
| Time on peritoneal dialysis (months) | 8 (6–20) | 14 (8–48) | 0.08 |
| Urine output (mL) | 70 (0–1000) | 500 (0–700) | 0.98 |
| Systolic blood pressure (mmHg) | 137 ± 25 | 133 ± 21 | 0.64 |
| Diastolic blood pressure (mmHg) | 89 ± 18 | 82 ± 14 | 0.22 |
| Body mass index (kg/m2) | 25.9 ± 4.0 | 27.0 ± 3.6 | 0.39 |
| Constipation, n (%) | 1 (7) | 12 (48) | 0.01 |
| Gastrointestinal symptoms (score) | 10 ± 2.1 | 12 ± 3.7 | 0.18 |
| Protein energy wasting, n (%) | 9 (64) | 15 (60) | 0.79 |
| Low muscle mass *, n (%) | 2 (14) | 8 (32) | 0.22 |
| Energy intake (kcal) | 1102 ± 355 | 1221 ± 397 | 0.36 |
| Protein intake (g) | 55 ± 28 | 57 ± 20 | 0.86 |
| Fiber intake (g) | 18 ± 6 | 18 ± 8 | 0.91 |
| Variable | Normal Cognitive Function (n = 14) | Mild Cognitive Impairment (n = 25) | p |
|---|---|---|---|
| Dialysis volume (L/day) | 10 (9.7–10.7) | 10 (9.6–10) | 0.77 |
| Ultrafiltration (mL/day) | 818 (534–1800) | 862 (371–1153) | 0.55 |
| Total Kt/Vurea (L/week) | 1.75 ± 0.59 | 1.89 ± 0.42 | 0.38 |
| Residual kidney function (mL/min) | 0.08 (0–3.1) | 1.3 (0–3.0) | 0.61 |
| nPNA (g/kg) | 0.82 ± 0.20 | 0.81 ± 0.13 | 0.89 |
| Hemoglobin (g/dL) | 11.3 ± 2.6 | 11.6 ± 2.44 | 0.73 |
| Glucose (mg/dL) | 98 ± 16 | 116 ± 48 | 0.10 |
| Urea (mg/dL) | 126 ± 31 | 120 ± 32 | 0.56 |
| Creatinine (mg/dL) | 14.9 ± 5.4 | 11.3 ± 3.7 | 0.02 |
| Phosphorus (mg/dL) | 5.9 ± 1.7 | 5.2 ± 1.2 | 0.15 |
| Calcium (mg/dL) | 8.3 ± 1.4 | 8.8 ± 0.7 | 0.40 |
| Potassium (mmol/L) | 4.6 ± 0.3 | 4.5 ± 0.6 | 0.58 |
| Sodium (mmol/L) | 141 ± 2.7 | 140 ± 3.1 | 0.19 |
| Total cholesterol (mg/dL) | 169 ± 53 | 176 ± 34 | 0.60 |
| Triglycerides (mg/dL) | 145 ± 95 | 132 ± 83 | 0.66 |
| Albumin (g/dL) | 4.03 ± 0.49 | 3.89 ± 0.41 | 0.38 |
| C-reactive protein (mg/L) | 0.85 (0.57–4.8) | 1.7 (0.50–7.3) | 0.50 |
| Lipopolysaccharides (ng/mL) | 58 (44–74) | 49 (33–87) | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-del-Campo, F.; Vega-Magaña, N.; Salazar-Félix, N.A.; Cueto-Manzano, A.M.; Peña-Rodríguez, M.; Cortés-Sanabria, L.; Romo-Flores, M.L.; Rojas-Campos, E. Gut Microbiome Is Related to Cognitive Impairment in Peritoneal Dialysis Patients. Nutrients 2024, 16, 2659. https://doi.org/10.3390/nu16162659
Martín-del-Campo F, Vega-Magaña N, Salazar-Félix NA, Cueto-Manzano AM, Peña-Rodríguez M, Cortés-Sanabria L, Romo-Flores ML, Rojas-Campos E. Gut Microbiome Is Related to Cognitive Impairment in Peritoneal Dialysis Patients. Nutrients. 2024; 16(16):2659. https://doi.org/10.3390/nu16162659
Chicago/Turabian StyleMartín-del-Campo, Fabiola, Natali Vega-Magaña, Noé A. Salazar-Félix, Alfonso M. Cueto-Manzano, Marcela Peña-Rodríguez, Laura Cortés-Sanabria, María L. Romo-Flores, and Enrique Rojas-Campos. 2024. "Gut Microbiome Is Related to Cognitive Impairment in Peritoneal Dialysis Patients" Nutrients 16, no. 16: 2659. https://doi.org/10.3390/nu16162659







