Seasonal Changes in Vitamin D-Effective UVB Availability in Europe and Associations with Population Serum 25-Hydroxyvitamin D
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of European Sites to Model UVB Availability
2.2. Generation of UV Dose Data for European Sites
2.3. Estimating the Cosine Solar Zenith Angle for European Sites
2.4. Standardized Serum 25-Hydroxyvitamin D Concentration for Four Selected Population Samples
2.5. Statistical Analysis
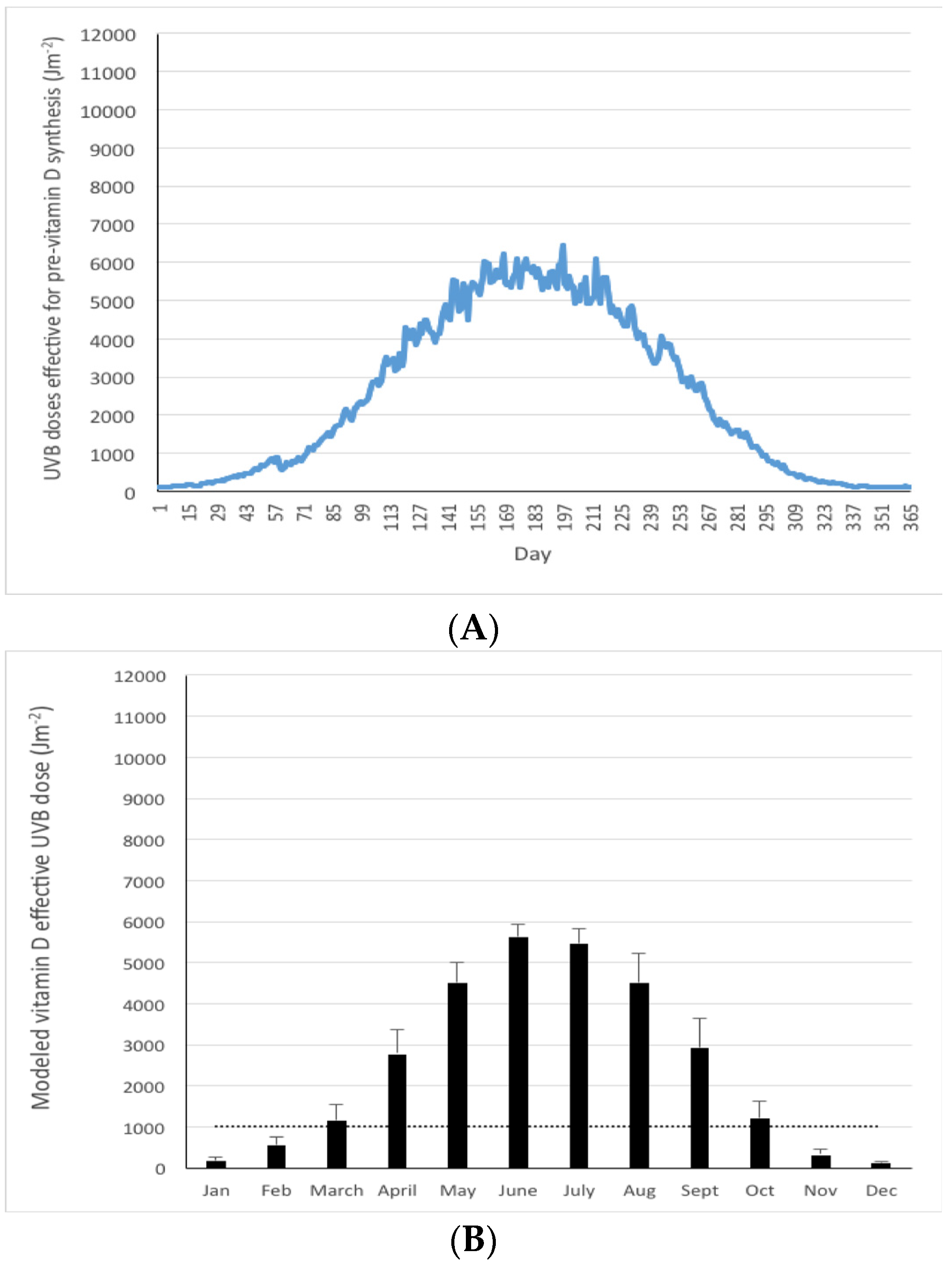
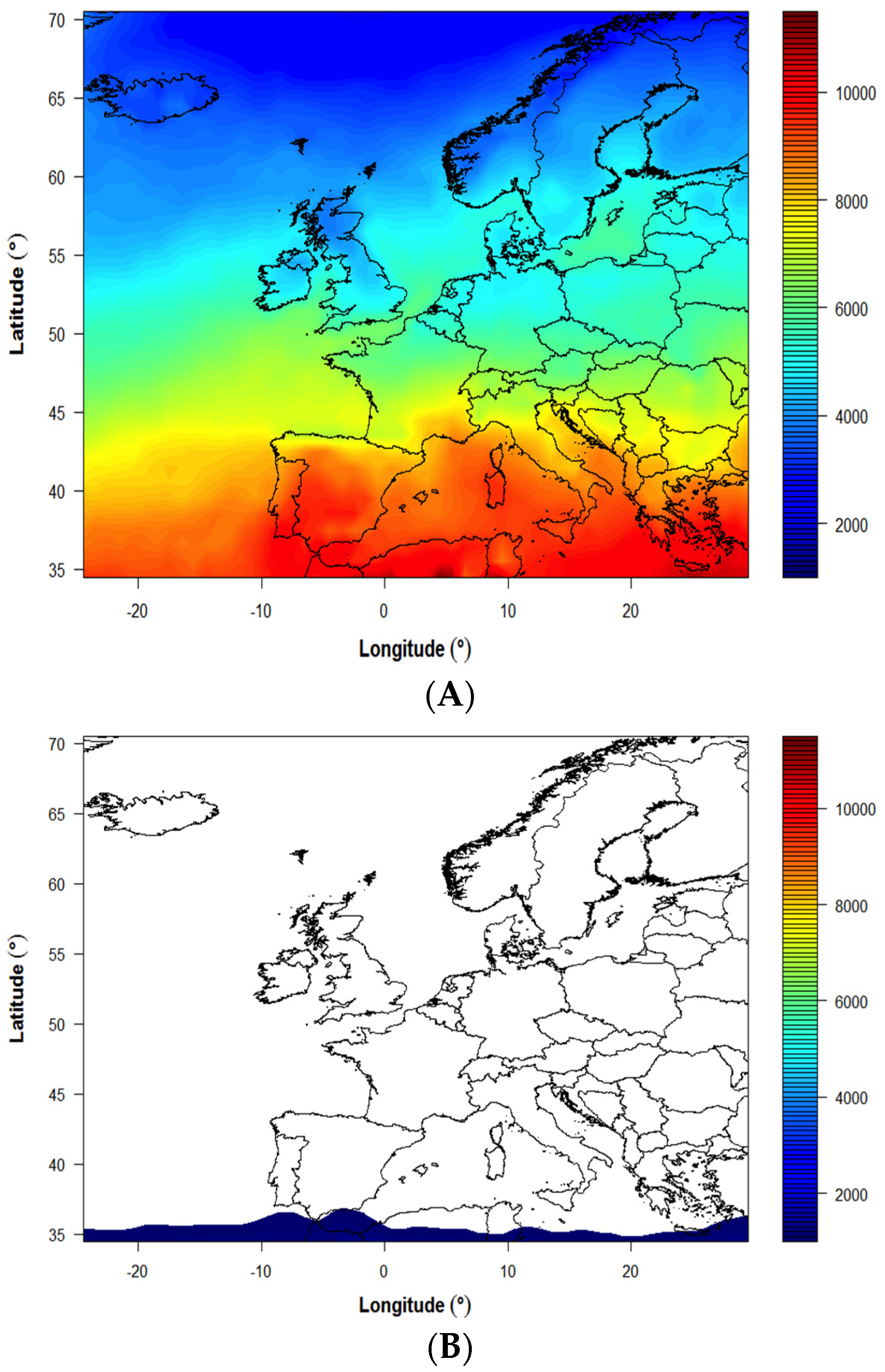
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kiely, M.; Cashman, K.D. The ODIN project: Development of food-based approaches for prevention of vitamin D deficiency throughout life. Nutr. Bull. 2015, 40, 235–246. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Skrabakova, Z.; Gonzalez-Gross, M.; Valtuena, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Molgaard, C.; et al. Vitamin D deficiency in Europe-Pandemic? Am. J. Clin Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Kimlin, M.G. Geographic location and vitamin D synthesis. Mol. Asp. Med. 2008, 29, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R. Who, what, where and when-influences on cutaneous vitamin D synthesis. Prog. Biophys. Mol. Biol. 2006, 92, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Worldwide status of vitamin D nutrition. J. Steroid Biochem. Mol. Biol. 2010, 121, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Hilger, J.; Friedel, A.; Herr, R.; Rausch, T.; Roos, F.; Wahl, D.A.; Pierroz, D.D.; Weber, P.; Hoffmann, K. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014, 111, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Sempos, C.T.; Vesper, H.W.; Phinney, K.W.; Thienpont, L.M.; Coates, P.M.; Vitamin, D.S.P. Vitamin D status as an international issue: National surveys and the problem of standardization. Scand. J. Clin Lab. Investig. Suppl. 2012, 72, 32–40. [Google Scholar]
- Cashman, K.D.; Dowling, K.G.; Skrabakova, Z.; Kiely, M.; Lamberg-Allardt, C.; Durazo-Arvizu, R.A.; Sempos, C.T.; Koskinen, S.; Lundqvist, A.; Sundvall, J.; et al. Standardizing serum 25-hydroxyvitamin D data from four Nordic population samples using the Vitamin D Standardization Program protocols: Shedding new light on vitamin D status in Nordic individuals. Scand. J. Clin. Lab. Investig. 2015, 75, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Kazantzidis, A.; Smedley, A.; Kift, R.; Rimmer, J.; Berry, J.L.; Rhodes, L.E.; Webb, A.R. A modeling approach to determine how much UV radiation is available across the UK and Ireland for health risk and benefit studies. Photochem. Photobiol. Sci. 2015, 14, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.; Kylling, A. Technical note: The libRadtran software package for radiative transfer calculations-description and examples of use. Atmos. Chem. Phys. 2005, 5, 1855–1877. [Google Scholar] [CrossRef]
- Tropospheric Emission Monitoring Internet Service (TEMIS). GTOPO30 Elevation Data at Different Resolutions Dataset. Available online: http://www.temis.nl/data/topo/dem2grid.html (accessed on 1 May 2016).
- Cashman, K.D.; Kazantzidis, A.; Webb, A.R.; Kiely, M. An Integrated Predictive Model of Population Serum 25-Hydroxyvitamin D for Application in Strategy Development for Vitamin D Deficiency Prevention. J. Nutr. 2015, 145, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Commission Internationale de L’Eclairage (CIE). Action spectrum for the production of previtamin D3 in human skin. Available online: http://www.cie.co.at/index.php/Publications/index.php?i_ca_id=450 (accessed on 29 August 2016).
- Van Dijk, A.; den Outer, P.; van Kranen, H.; Slaper, H. The action spectrum for vitamin D3: Initial skin reaction and prolonged exposure. Photochem. Photobiol. Sci. 2016, 15, 896–909. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R.; Kline, L.; Holick, M.F. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J. Clin. Endocrinol. Metab. 1988, 67, 373–378. [Google Scholar] [CrossRef] [PubMed]
- NOAA ESRL Solar Position Calculator. Available online: http://www.esrl.noaa.gov/gmd/grad/solcalc/azel.html (accessed on 1 May 2016).
- Eggen, A.E.; Mathiesen, E.B.; Wilsgaard, T.; Jacobsen, B.K.; Njolstad, I. The sixth survey of the Tromso Study (Tromso 6) in 2007–08: Collaborative research in the interface between clinical medicine and epidemiology: Study objectives, design, data collection procedures, and attendance in a multipurpose population-based health survey. Scand. J. Public Health 2013, 41, 65–80. [Google Scholar] [PubMed]
- Oberg, J.; Jorde, R.; Almas, B.; Emaus, N.; Grimnes, G. Vitamin D deficiency and lifestyle risk factors in a Norwegian adolescent population. Scand. J. Public Health 2014, 42, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.B.; Launer, L.J.; Eiriksdottir, G.; Kjartansson, O.; Jonsson, P.V.; Sigurdsson, G.; Thorgeirsson, G.; Aspelund, T.; Garcia, M.E.; Cotch, M.F.; et al. Age, Gene/Environment Susceptibility-Reykjavik Study: Multidisciplinary applied phenomics. Am. J. Epidemiol. 2007, 165, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Irish Universities Nutrition Alliance (IUNA). National Adult Nutrition Survey: Summary Report. 2011. Available online: http://www.iuna.net/wp-content/uploads/2010/12/National-Adult-Nutrition-Survey-Summary-Report-March-2011.pdf (accessed on 4 January 2016).
- Cashman, K.D.; Kiely, M.; Kinsella, M.; Durazo-Arvizu, R.A.; Tian, L.; Zhang, Y.; Lucey, A.; Flynn, A.; Gibney, M.J.; Vesper, H.W.; et al. Evaluation of Vitamin D Standardization Program protocols for standardizing serum 25-hydroxyvitamin D data: A case study of the program's potential for national nutrition and health surveys. Am. J. Clin. Nutr. 2013, 97, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Vitamin D Standardization Certification Program. Available online: https://www.cdc.gov/labstandards/pdf/hs/CDC_Certified_Vitamin_D_Procedures.pdf (accessed on 1 July 2016).
- Fioletov, V.E.; McArthur, L.J.; Mathews, T.W.; Marrett, L. Estimated ultraviolet exposure levels for a sufficient vitamin D status in North America. J. Photochem. Photobiol. B 2010, 100, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Rabenberg, M.; Scheidt-Nave, C.; Busch, M.A.; Rieckmann, N.; Hintzpeter, B.; Mensink, G.B.M. Vitamin D status among adults in Germany—Results from the German Health Interview and Examination Survey for Adults (DEGS1). BMC Public Health 2015, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hypponen, E.; Power, C. Hypovitaminosis D in British adults at age 45 y: Nationwide cohort study of dietary and lifestyle predictors. Am. J. Clin. Nutr. 2007, 85, 860–868. [Google Scholar] [PubMed]
- Jablonski, N.G.; Chaplin, G. Human skin pigmentation, migration and disease susceptibility. Philos Trans. R Soc. Lond B Biol. Sci. 2012, 367, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Black, L.J.; Walton, J.; Flynn, A.; Cashman, K.D.; Kiely, M. Small increments in Vitamin D intake by Irish adults over a decade show that strategic initiatives to fortify the food supply are needed. J. Nutr. 2015, 145, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Thorgeirsdottir, H.; Valgeirsdottir, H.; Gunnarsdottir, I.; Gisladottir, E.; Gunnarsdottir, B.E.; Thorsdottir, I. What do Icelanders Eat? Dietary Survey on the Diet of Icelanders 2010–2011. Main Results; Directorate of Health, Icelandic Food and Veterinary Authority, Unit for nutrition research Landspitali—The National University Hospital of Iceland and University of Iceland: Reykjavik, Iceland, 2011. [Google Scholar]
- Norwegian Directorate of Health, Norwegian Food Safety Authority and University of Oslo. Norkost 3. A Nationwide Dietary Survey among Men and Women in Norway Aged 18–70 Years; Report IS-2000; Directorate of Health: Oslo, Norwegian, May 2012. [Google Scholar]
- Brustad, M.; Alsaker, E.; Engelsen, O.; Aksnes, L.; Lund, E. Vitamin D status of middle-aged women at 65–71 degrees N in relation to dietary intake and exposure to ultraviolet radiation. Public Health Nutr. 2004, 7, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Lamberg-Allardt, C.; Brustad, M.; Meyer, H.E.; Steingrimsdottir, L. Vitamin D—A systematic literature review for the 5th edition of the Nordic Nutrition Recommendations. Food Nutr. Res. 2013, 3, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamycheva, E.; Joakimsen, R.M.; Jorde, R. Intakes of calcium and vitamin D predict body mass index in the population of Northern Norway. J. Nutr. 2003, 133, 102–106. [Google Scholar] [PubMed]
- Johansson, L.S.K. Norkost 1997—Nationally Representative Dietary Survey in Men and Women Aged 16–79 Years (Norkost 1997–Landsomfattende Kostholdsundersøkelse Blant Mennog kviner i alderen 16–79 år); National Council for Nutrition and Physical Activity: Oslo, Norway, 1999. [Google Scholar]
- Didriksen, A.; Burild, A.; Jakobsen, J.; Fuskevag, O.M.; Jorde, R. Vitamin D3 increases in abdominal subcutaneous fat tissue after supplementation with vitamin D3. Eur. J. Endocrinol. 2015, 172, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Abboud, M.; Gordon-Thomson, C.; Hoy, A.J.; Balaban, S.; Rybchyn, M.S.; Cole, L.; Su, Y.; Brennan-Speranza, T.C.; Fraser, D.R.; Mason, R.S. Uptake of 25-hydroxyvitamin D by muscle and fat cells. J. Steroid Biochem. Mol. Biol. 2014, 144, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.I.; Weiderpass, E. Lung cancer mortality trends in 36 European countries: Secular trends and birth cohort patterns by sex and region 1970–2007. Int. J. Cancer 2010, 126, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Brot, C.; Jorgensen, N.R.; Sorensen, O.H. The influence of smoking on vitamin D status and calcium metabolism. Eur. J. Clin. Nutr. 1999, 53, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Øverby, N.C.A. UNGKOST-2000—Nationally Representative Dietary Survey in Norwegian 4th and 8th Graders (UNGKOST-2000—Landsomfattende Kostholdsundersøkelse Blant Elever i 4-og 8. Klasse i Norge); The Norwegian Directorate of Health: Oslo, Norway, 2002. [Google Scholar]
- Norwegian Food Control Authority. Kostraod—Fiskelever Food Advice—Fish. Available online: http://www. snt.no/nytt/kosthold/fisk_skalldyr/fiskelever.htm (accessed on 22 June 2016).
- Ovesen, L.; Brot, C.; Jakobsen, J. Food contents and biological activity of 25-hydroxyvitamin D: A vitamin D metabolite to be reckoned with? Ann. Nutr. Metab. 2003, 47, 107–113. [Google Scholar] [CrossRef] [PubMed]


| Country/Region | Latitude (° N) | Coordinates for Modeling | Modeled Yearly UVB (Jm−2) * | Vitamin D Winter ** (months) | % of the Year <1000 Jm−2 | Cosine of Solar Zenith Angle *** | |
|---|---|---|---|---|---|---|---|
| Mean | SD | ||||||
| Greece: | |||||||
| Crete | 35 | 35.2° N, 24.9° E | 5500 | 3600 | 0 | 7 | 0.95 |
| Athens | 37 | 38.0° N, 23.7° E | 4800 | 3400 | 2 | 16 | 0.94 |
| Thessaloniki | 40 | 40.6° N, 22.9° E | 4100 | 3000 | 2 | 21 | 0.93 |
| Germany | 47–55 | Grid 1: 47.4 to 49.9° N, 6.4° E to 13.8° E; Grid 2: 50.0 to 51.9° N, 6°0′ E to 15°0′ E; Grid 3: 52.0 to 54.4° N, 7°0′ E to 14.7° E | 2500 | 2100 | 4 | 40 | 0.87 |
| Ireland | 51–54 | 51.4–54.5° N,5.4–10.5° W | 2100 | 1900 | 5 | 43 | 0.85 |
| UK | 50–59 | 50.5–58.0° N,4°5′ W–1°2′ E | 2000 | 1800 | 6 | 43 | 0.83 |
| Netherlands | 52 | 52.3° N to 52.6° N, 4.9°–5.1° E | 2200 | 2000 | 5 | 42 | 0.86 |
| Denmark: | |||||||
| Copenhagen | 56 | 54°45′ N to 55°51′ N, 11° 51′ E to 12°30′ E | 2000 | 1900 | 6 | 45 | 0.83 |
| Aarhus | 56 | 56.2° N, 10.2° E | 1900 | 1800 | 6 | 48 | 0.82 |
| Finland | 60–70 | Grid 1: 59.8° to 63.8° N, 21.2° E to 30.5° E Grid 2: 63.9° N to 66.7° N, 23.7° E to 30°0′ E. | 1400 | 1600 | 6 | 55 | 0.75 |
| Iceland, Reykjavik | 64 | 64° 09′ N, 21° 57′ W | 1200 | 1400 | 7 | 60 | 0.76 |
| Norway: | |||||||
| Oslo | 60 | 59.9° N, 10.7° E | 1700 | 1700 | 6 | 51 | 0.79 |
| Tromsø | 69 | 69.4° N, 18.6° E | 900 | 1200 | 8 | 64 | 0.68 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Neill, C.M.; Kazantzidis, A.; Ryan, M.J.; Barber, N.; Sempos, C.T.; Durazo-Arvizu, R.A.; Jorde, R.; Grimnes, G.; Eiriksdottir, G.; Gudnason, V.; et al. Seasonal Changes in Vitamin D-Effective UVB Availability in Europe and Associations with Population Serum 25-Hydroxyvitamin D. Nutrients 2016, 8, 533. https://doi.org/10.3390/nu8090533
O’Neill CM, Kazantzidis A, Ryan MJ, Barber N, Sempos CT, Durazo-Arvizu RA, Jorde R, Grimnes G, Eiriksdottir G, Gudnason V, et al. Seasonal Changes in Vitamin D-Effective UVB Availability in Europe and Associations with Population Serum 25-Hydroxyvitamin D. Nutrients. 2016; 8(9):533. https://doi.org/10.3390/nu8090533
Chicago/Turabian StyleO’Neill, Colette M., Andreas Kazantzidis, Mary J. Ryan, Niamh Barber, Christopher T. Sempos, Ramon A. Durazo-Arvizu, Rolf Jorde, Guri Grimnes, Gudny Eiriksdottir, Vilmundur Gudnason, and et al. 2016. "Seasonal Changes in Vitamin D-Effective UVB Availability in Europe and Associations with Population Serum 25-Hydroxyvitamin D" Nutrients 8, no. 9: 533. https://doi.org/10.3390/nu8090533
APA StyleO’Neill, C. M., Kazantzidis, A., Ryan, M. J., Barber, N., Sempos, C. T., Durazo-Arvizu, R. A., Jorde, R., Grimnes, G., Eiriksdottir, G., Gudnason, V., Cotch, M. F., Kiely, M., Webb, A. R., & Cashman, K. D. (2016). Seasonal Changes in Vitamin D-Effective UVB Availability in Europe and Associations with Population Serum 25-Hydroxyvitamin D. Nutrients, 8(9), 533. https://doi.org/10.3390/nu8090533






