Seabuckthorn Leaves Extract and Flavonoid Glycosides Extract from Seabuckthorn Leaves Ameliorates Adiposity, Hepatic Steatosis, Insulin Resistance, and Inflammation in Diet-Induced Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Seabuckthorn Leaves (SL) Extract and Flavonoid Glycosides Extract from SL (SLG)
2.2. Experimental Animals and Diets
2.3. Energy Expenditure
2.4. Morphology of the Liver and Fat Tissues
2.5. Plasma Biomarkers
2.6. Fasting Blood Glucose, Intraperitoneal Glucose Tolerance Test, and Homeostatic Index of Insulin Resistance
2.7. Hepatic and Fecal Lipid Contents
2.8. Preparation of Hepatic Subcellular Fractions
2.9. Glucose- and Lipid-Regulating Enzyme Activity
2.10. Analysis of Gene Expression
2.11. Primer
2.12. Statistical Analysis
3. Results and Discussion
3.1. SL and SLG Supplement Lowered Body Weight Gain and Improved Plasma Lipid Profiles in DIO Mice
3.2. SL and SLG Supplement Lowered Adiposity by Decreasing Lipogenesis in Adipose Tissue, While Increasing Energy Expenditure in DIO Mice
3.3. SL and SLG Supplement Lowered the Levels of Hepatic Lipids and Lipotoxicity Markers by Modulating Hepatic Lipid Regulating Enzume Activities and Gene Expressions, and Increasing Fecal Lipids in DIO Mice
3.4. SL and SLG Improved Insulin Resistance and Glucose Tolerance by Modulating Activities of Hepatic
Glucose-Regulating Enzymes and Levels of Plasma Adipokines and Cytokines in DIO Mice
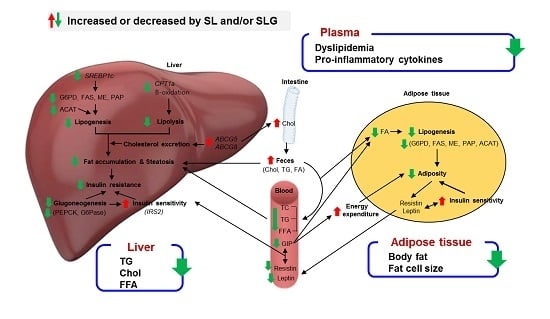
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABCG | ATP-binding cassette sub-family G member |
| ACAT | acyl-CoA:cholesterolacyltransferase |
| AI | atherogenic index |
| Apo | apolipoprotein |
| BW | body weight |
| CPT1α | carnitine palmitoyltransferase 1α |
| DIO | diet-induced obese |
| FAS | fatty acid synthase |
| FFA | free fatty acid |
| G6Pase | glucokinase, glucose-6-phosphatase |
| G6PD | glucose-6-phosphate dehydrogenase |
| GIP | incretin hormone gastric inhibitory polypeptide |
| GIPR | GIP receptor |
| HFD | high-fat diet |
| HOMA-IR | homeostasis model assessment-estimated insulin resistance |
| IL | interleukin |
| IPGTT | intraperitoneal glucose tolerance test |
| IRS2 | insulin receptor substrate 2 |
| ME | malic enzyme |
| ND | normal diet |
| PAI-1 | plasminogen activator inhibitor-1 |
| PAP | phosphatidate phosphohydrolase |
| PEPCK | phosphoenolpyruvate carboxykinase |
| SL | seabuckthorn leaf |
| SLG | flavonoid glycosides from seabuckthorn leaves |
| SREBP1c | sterol regulatory element-binding protein 1c |
| T2DM | type 2 diabetes mellitus |
| TNF-α | tumor necrosis factor α |
| WAT | white adipose tissue |
References
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.; Catalano, K.J.; Ananthnarayan, S.; Kim, S.P.; Van Citters, G.W.; Dea, M.K.; Bergman, R.N. Molecular evidence supporting the portal theory: A causative link between visceral adiposity and hepatic insulin resistance. Am. J. Physiol. Endocrinol. MeTable 2005, 288, E454–E461. [Google Scholar] [CrossRef] [PubMed]
- Suomela, J.P.; Ahotupa, M.; Yang, B.; Vasankari, T.; Kallio, H. Absorption of flavonols derived from sea buckthorn (Hippophaë rhamnoides L.) and their effect on emerging risk factors for cardiovascular disease in humans. J. Agric. Food Chem. 2006, 54, 7364–7369. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Ganju, L.; Katiyal, A.; Padwad, Y.; Mishra, K.P.; Chanda, S.; Karan, D.; Yogendra, K.M.; Sawhney, R.C. Effect of Hippophae rhamnoides leaf extract against Dengue virus infection in human blood-derived macrophages. Phytomedicine 2008, 15, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Geetha, S.; Sai Ram, M.; Singh, V.; Ilavazhagan, G.; Sawhney, R.C. Anti-oxidant and immunomodulatory properties of seabuckthorn (Hippophae rhamnoides)—An in vitro study. J. Ethnopharmacol. 2002, 79, 373–378. [Google Scholar] [CrossRef]
- Ganju, L.; Padwad, Y.; Singh, R.; Karan, D.; Chanda, S.; Chopra, M.K.; Bhatnagar, P.; Kashyap, R.; Sawhney, R.C. Anti-inflammatory activity of Seabuckthorn (Hippophae rhamnoides) leaves. Int. Immunopharmacol. 2005, 5, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, E.; Lee, J.; Kim, S.; Huh, S.; Kim, Y.; Kim, Y.; Byun, S.Y.; Kim, Y.S.; Park, D. Isorhamnetin represses adipogenesis in 3T3-L1 cells. Obesity (Silver Spring) 2009, 17, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.X.; Li, X.; Sun, C.D.; Chen, K.S. Hypoglycemic and hypolipidemic effects of quercetin and its glycosides. Zhongguo Zhong Yao Za Zhi 2015, 40, 4560–4567. [Google Scholar] [PubMed]
- Kim, J.S.; Kwon, Y.S.; Sa, Y.J.; Kim, M.J. Isolation and identification of sea buckthorn (Hippophae rhamnoides) phenolics with antioxidant activity and α-glucosidase inhibitory effect. J. Agric. Food Chem. 2011, 59, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloan-Stanley, G.H. A simple method for isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Davidson, A.L.; Arion, W.J. Factors underlying significant underestimations of glucokinase activity in crude liver extracts: Physiological implications of higher cellular activity. Arch. Biochem. Biophys. 1987, 253, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Rudack, D.; Chisholm, E.M.; Holten, D. Rat liver glucose 6-phosphate dehydrogenase. Regulation by carbohydrate diet and insulin. J. Biol. Chem. 1971, 246, 1249–1254. [Google Scholar] [PubMed]
- Lazarow, P.B. Assay of peroxisomal beta-oxidation of fatty acids. Methods Enzymol. 1981, 72, 315–319. [Google Scholar] [PubMed]
- Ochoa, S. Malic enzyme: Malic enzymes from pigeon and wheat germ. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA, 1955; Volume 1, pp. 323–326. [Google Scholar]
- Walton, P.A.; Possmayer, F. Mg2-dependent phosphatidate phosphohydrolase of rat lung: Development of an assay employing a defined chemical substrate which reflects the phosphohydrolase activity measured using membrane-bound substrate. Anal. Biochem. 1985, 151, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Alegre, M.; Ciudad, C.J.; Fillat, C.; Guinovart, J.J. Determination of glucose-6-phosphatase activity using the glucose dehydrogenase-coupled reaction. Anal. Biochem. 1988, 173, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Bentle, L.A.; Lardy, H.A. Interaction of anions and divalent metal ions with phosphoenolpyruvate carboxykinase. J. Biol. Chem. 1976, 251, 2916–2921. [Google Scholar] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.I.; Kim, M.S.; Lee, K.M.; Park, S.K.; Seo, K.I.; Kim, H.J.; Kim, M.J.; Choi, M.S.; Lee, M.K. Anti-visceral obesity and antioxidant effects of powdered sea buckthorn (Hippophae rhamnoides L.) leaf tea in diet-induced obese mice. Food Chem. Toxicol. 2011, 49, 2370–2376. [Google Scholar] [CrossRef] [PubMed]
- Pichiah, P.B.; Moon, H.J.; Park, J.E.; Moon, Y.J.; Cha, Y.S. Ethanolic extract of seabuckthorn (Hippophae rhamnoides L.) prevents high-fat diet-induced obesity in mice through down-regulation of adipogenic and lipogenic gene expression. Nutr. Res. 2012, 32, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Banerji, M.A.; Buckley, M.C.; Chaiken, R.L.; Gordon, D.; Lebovitz, H.E.; Kral, J.G. Liver fat, serum triglycerides and visceral adipose tissue in insulin-sensitive and insulin-resistant black men with NIDDM. Int. J. Obes. Relat. Metab. Disord. 1995, 19, 846–850. [Google Scholar] [PubMed]
- Drucker, D.J. The biology of incretin hormones. Cell. MeTable 2006, 3, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Taminato, T.; Seino, Y.; Goto, Y.; Inoue, Y.; Kadowaki, S. Synthetic gastric inhibitory polypeptide. Stimulatory effect on insulin and glucagon secretion in the rat. Diabetes 1977, 26, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.D.; Larsen, M.O.; Winzell, M.S.; Jelic, K.; Lindgren, O.; Deacon, C.F.; Ahrén, B. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am. J. Physiol. Endocrinol. MeTable 2008, 295, E779–E784. [Google Scholar] [CrossRef] [PubMed]
- Creutzfeldt, W.; Ebert, R.; Willms, B.; Frerichs, H.; Brown, J.C. Gastric inhibitory polypeptide (GIP) and insulin in obesity: Increased response to stimulation and defective feedback control of serum levels. Diabetologia 1978, 14, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yamada, Y.; Tsukiyama, K.; Miyawaki, K.; Hosokawa, M.; Nagashima, K.; Toyoda, K.; Naitoh, R.; Mizunoya, W.; Fushiki, T.; et al. Gastric inhibitory polypeptide modulates adiposity and fat oxidation under diminished insulin action. Biochem. Biophys. Res. Commun. 2005, 335, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Nian, C.; McIntosh, C.H. Resistin is a key mediator of glucose-dependent insulinotropic polypeptide (GIP) stimulation of lipoprotein lipase (LPL) activity in adipocytes. J. Biol. Chem. 2007, 282, 34139–34147. [Google Scholar] [CrossRef]
- Kim, S.J.; Nian, C.; McIntosh, C.H. Activation of lipoprotein lipase by glucose-dependent insulinotropic polypeptide in adipocytes. A role for a protein kinase B, LKB1, and AMP-activated protein kinase cascade. J. Biol. Chem. 2007, 282, 8557–8567. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Lazar, M.A. The current biology of resistin. J. Intern. Med. 2004, 255, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, M.; Verhelst, X.; Geerts, A.; Lapauw, B.; Calders, P. Association of recently described adipokines with liver histology in biopsy-proven non-alcoholic fatty liver disease: A systematic review. Obes. Rev. 2016, 17, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Aronis, K.N.; Kountouras, J.; Raptis, D.D.; Vasiloglou, M.F.; Mantzoros, C.S. Circulating leptin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Diabetologia 2016, 59, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Zhao, C.Y.; Wang, W.; Wang, Y.D.; Sun, H.; Cao, W.; Yu, W.Y.; Zhang, L.; Ji, R.; Li, M.; Gao, J. The relationship between hepatic resistin overexpression and inflammation in patients with nonalcoholic steatohepatitis. BMC Gastroenterol. 2014, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- López-Jaramillo, P.; Gómez-Arbeláez, D.; López-López, J.; López-López, C.; Martínez-Ortega, J.; Gómez-Rodríguez, A.; Triana-Cubillos, S. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm. Mol. Biol. Clin. Investig. 2014, 18, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Nguyen, K.D.; Goh, Y.P. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011, 11, 738–749. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, I.; Rodríguez-Juan, C.; Díaz-Sanjuan, T.; del Hoyo, P.; Colina, F.; Muñoz-Yagüe, T.; Solís-Herruzo, J.A. Uric acid and anti-TNF antibody improve mitochondrial dysfunction in ob/ob mice. Hepatology 2006, 44, 581–591. [Google Scholar] [CrossRef] [PubMed]
- McCullough, A.J. Pathophysiology of nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2006, 40, S17–S29. [Google Scholar] [CrossRef] [PubMed]
- Spranger, J.; Kroke, A.; Möhlig, M.; Hoffmann, K.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003, 52, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Nov, O.; Shapiro, H.; Ovadia, H.; Tarnovscki, T.; Dvir, I.; Shemesh, E.; Kovsan, J.; Shelef, I.; Carmi, Y.; Voronov, E.; et al. Interleukin-1β regulates fat-liver crosstalk in obesity by auto-paracrine modulation of adipose tissue inflammation and expandability. PLoS ONE 2013, 8, e53626. [Google Scholar] [CrossRef] [PubMed]
- Bastard, J.P.; Maachi, M.; Van Nhieu, J.T.; Jardel, C.; Bruckert, E.; Grimaldi, A.; Robert, J.J.; Capeau, J.; Hainque, B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J. Clin. Endocrinol. MeTable 2002, 87, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Juhan-Vague, I.; Alessi, M.C.; Mavri, A.; Morange, P.E. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J. Thromb. Haemost. 2003, 1, 1575–1579. [Google Scholar] [CrossRef] [PubMed]





| Position | Isorhamnetin 3-Glucoside | Quercetin 3-Glucoside |
|---|---|---|
| 1H-NMR | ||
| 6 | 6.43 (H, br s) | 6.45 (H, d, J = 2.4 Hz) |
| 8 | 6.72 (H, br s) | 6.73 (H, d, J = 2.4 Hz) |
| 2′ | 7.92 (H, d, J = 1.3 Hz) | 7.71 (H, d, J = 1.8 Hz) |
| 3′ | - | - |
| 5′ | 6.88 (H, d, J = 8.4 Hz) | 6.85 (H, d, J = 7.8 Hz) |
| 6′ | 7.60 (H, dd, J = 1.3 & 8.9 Hz) | 7.60 (H, dd, J = 1.8 & 7.8 Hz) |
| Glu 1” | 5.44 (H, d, J = 6.6 Hz) | 5.20 (H, d, J = 7.2 Hz) |
| 2”~6” | 3.18 ~ 3.70 | 3.21 ~ 3.72 |
| OCH3 | 3.93 (3H, s) | |
| 13C-NMR | ||
| 2 | 157.57 | 158.00 |
| 3 | 135.32 | 135.72 |
| 4 | 177.79 | 179.68 |
| 5 | 161.71 | 162.85 |
| 6 | 100.06 | 100.58 |
| 7 | 165.12 | 163.63 |
| 8 | 94.95 | 95.47 |
| 9 | 158.63 | 159.60 |
| 10 | 105.66 | 105.03 |
| 1′ | 123.12 | 122.54 |
| 2′ | 114.38 | 116.13 |
| 3′ | 148.43 | 146.18 |
| 4′ | 150.88 | 150.68 |
| 5′ | 116.07 | 117.63 |
| 6′ | 123.81 | 122.54 |
| Glu 1 | 103.68 | 103.94 |
| 2 | 75.94 | 75.74 |
| 3 | 78.12 | 77.25 |
| 4 | 71.50 | 70.08 |
| 5 | 78.55 | 75.05 |
| 6 | 62.59 | 62.57 |
| OCH3 | 56.77 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, E.-Y.; Lee, J.; Kim, Y.J.; Do, A.; Choi, J.-Y.; Cho, S.-J.; Jung, U.J.; Lee, M.-K.; Park, Y.B.; Choi, M.-S. Seabuckthorn Leaves Extract and Flavonoid Glycosides Extract from Seabuckthorn Leaves Ameliorates Adiposity, Hepatic Steatosis, Insulin Resistance, and Inflammation in Diet-Induced Obesity. Nutrients 2017, 9, 569. https://doi.org/10.3390/nu9060569
Kwon E-Y, Lee J, Kim YJ, Do A, Choi J-Y, Cho S-J, Jung UJ, Lee M-K, Park YB, Choi M-S. Seabuckthorn Leaves Extract and Flavonoid Glycosides Extract from Seabuckthorn Leaves Ameliorates Adiposity, Hepatic Steatosis, Insulin Resistance, and Inflammation in Diet-Induced Obesity. Nutrients. 2017; 9(6):569. https://doi.org/10.3390/nu9060569
Chicago/Turabian StyleKwon, Eun-Young, Jeonghyeon Lee, Ye Jin Kim, Ara Do, Ji-Young Choi, Su-Jung Cho, Un Ju Jung, Mi-Kyung Lee, Yong Bok Park, and Myung-Sook Choi. 2017. "Seabuckthorn Leaves Extract and Flavonoid Glycosides Extract from Seabuckthorn Leaves Ameliorates Adiposity, Hepatic Steatosis, Insulin Resistance, and Inflammation in Diet-Induced Obesity" Nutrients 9, no. 6: 569. https://doi.org/10.3390/nu9060569
APA StyleKwon, E.-Y., Lee, J., Kim, Y. J., Do, A., Choi, J.-Y., Cho, S.-J., Jung, U. J., Lee, M.-K., Park, Y. B., & Choi, M.-S. (2017). Seabuckthorn Leaves Extract and Flavonoid Glycosides Extract from Seabuckthorn Leaves Ameliorates Adiposity, Hepatic Steatosis, Insulin Resistance, and Inflammation in Diet-Induced Obesity. Nutrients, 9(6), 569. https://doi.org/10.3390/nu9060569