A Complex In Vitro Degradation Study on Polydioxanone Biliary Stents during a Clinically Relevant Period with the Focus on Raman Spectroscopy Validation
Abstract
:1. Introduction
2. Materials and Methods
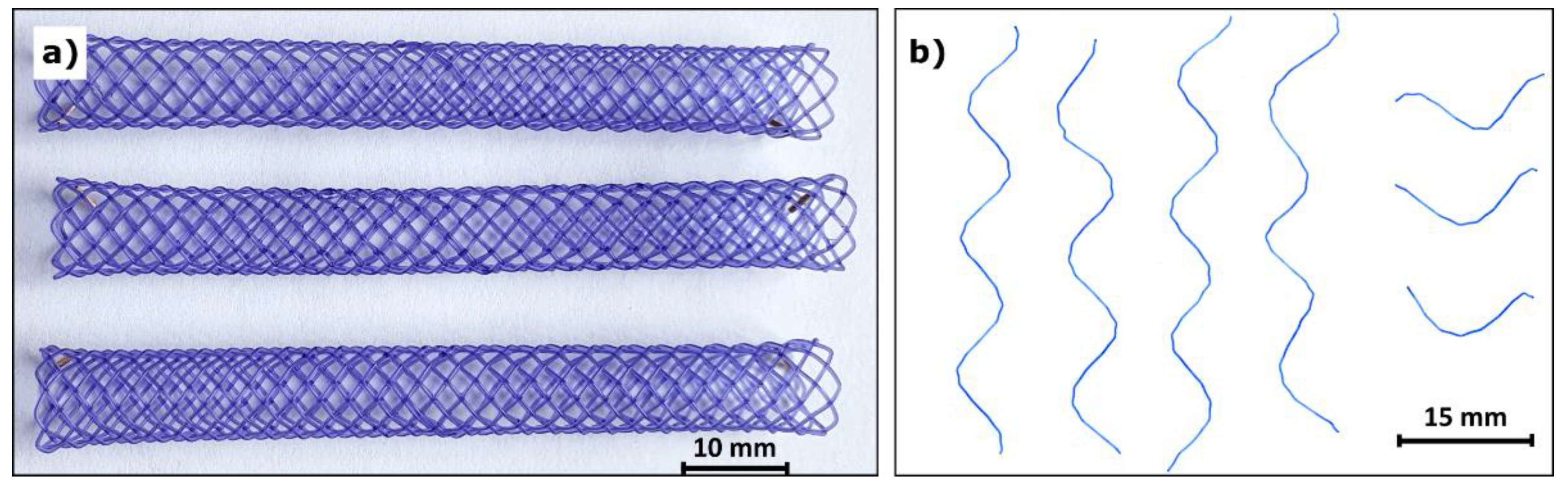
2.1. Stent Degradation
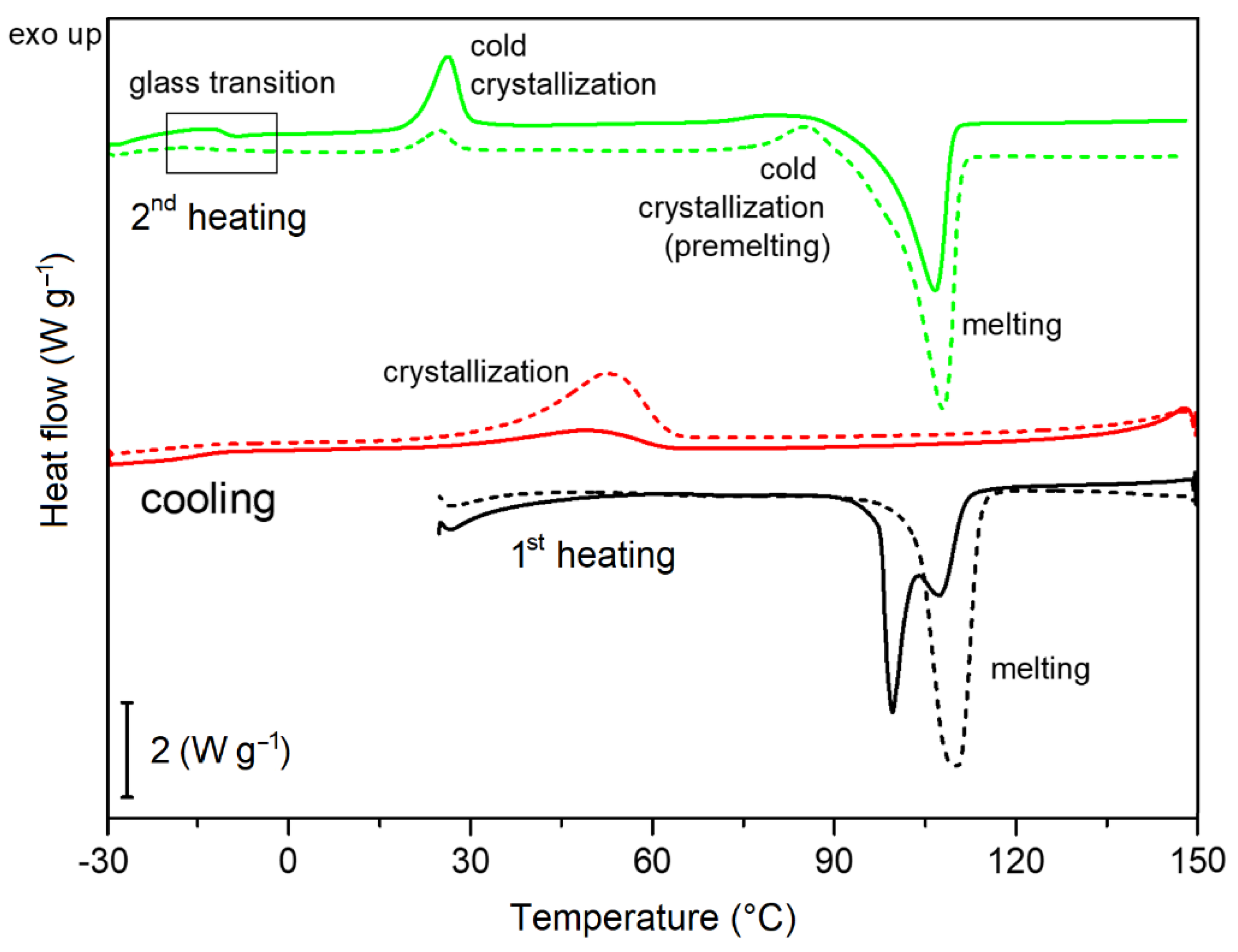
2.2. Thermal Analysis
2.3. Scanning Electron Microscopy
2.4. Infrared Spectroscopy
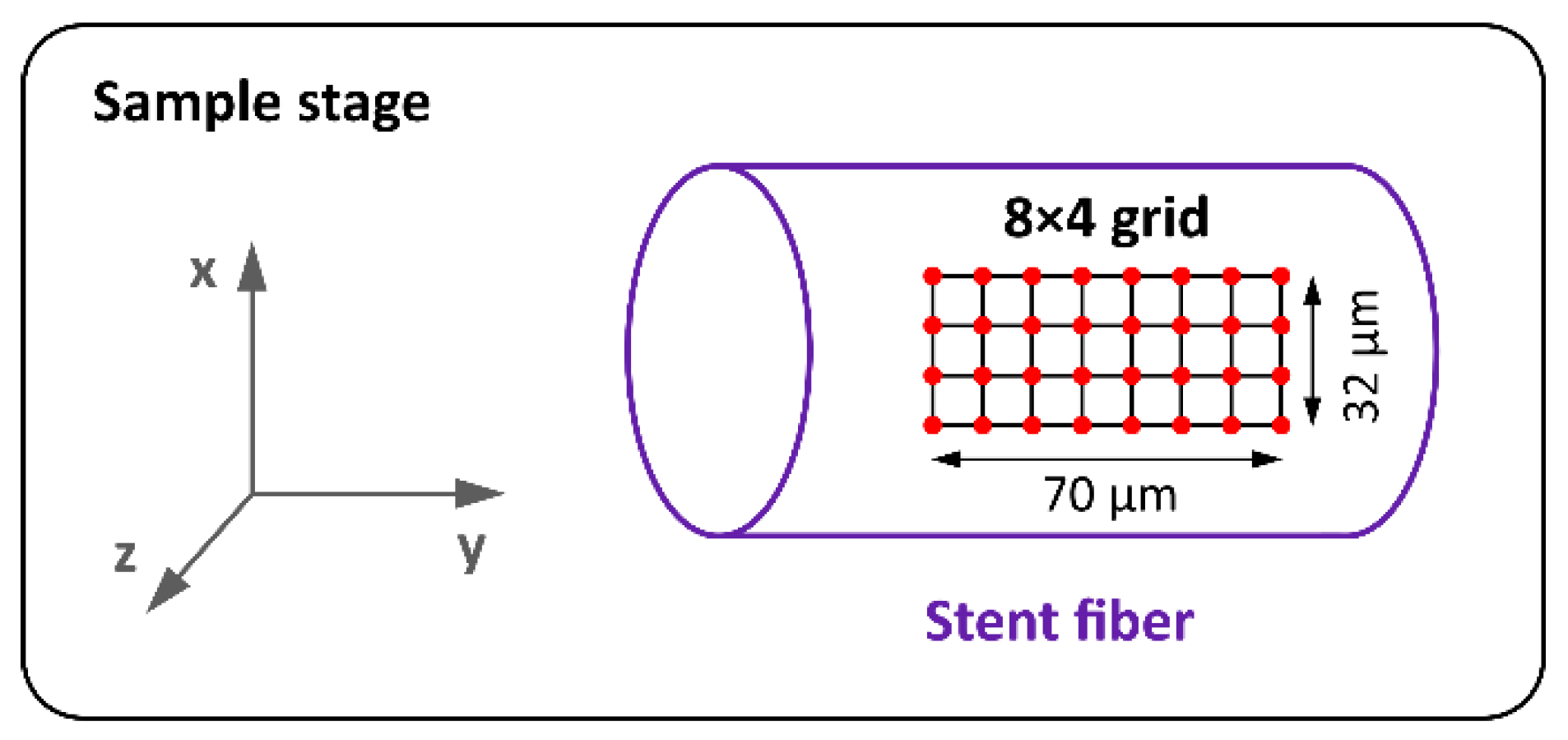
2.5. Raman Spectroscopy
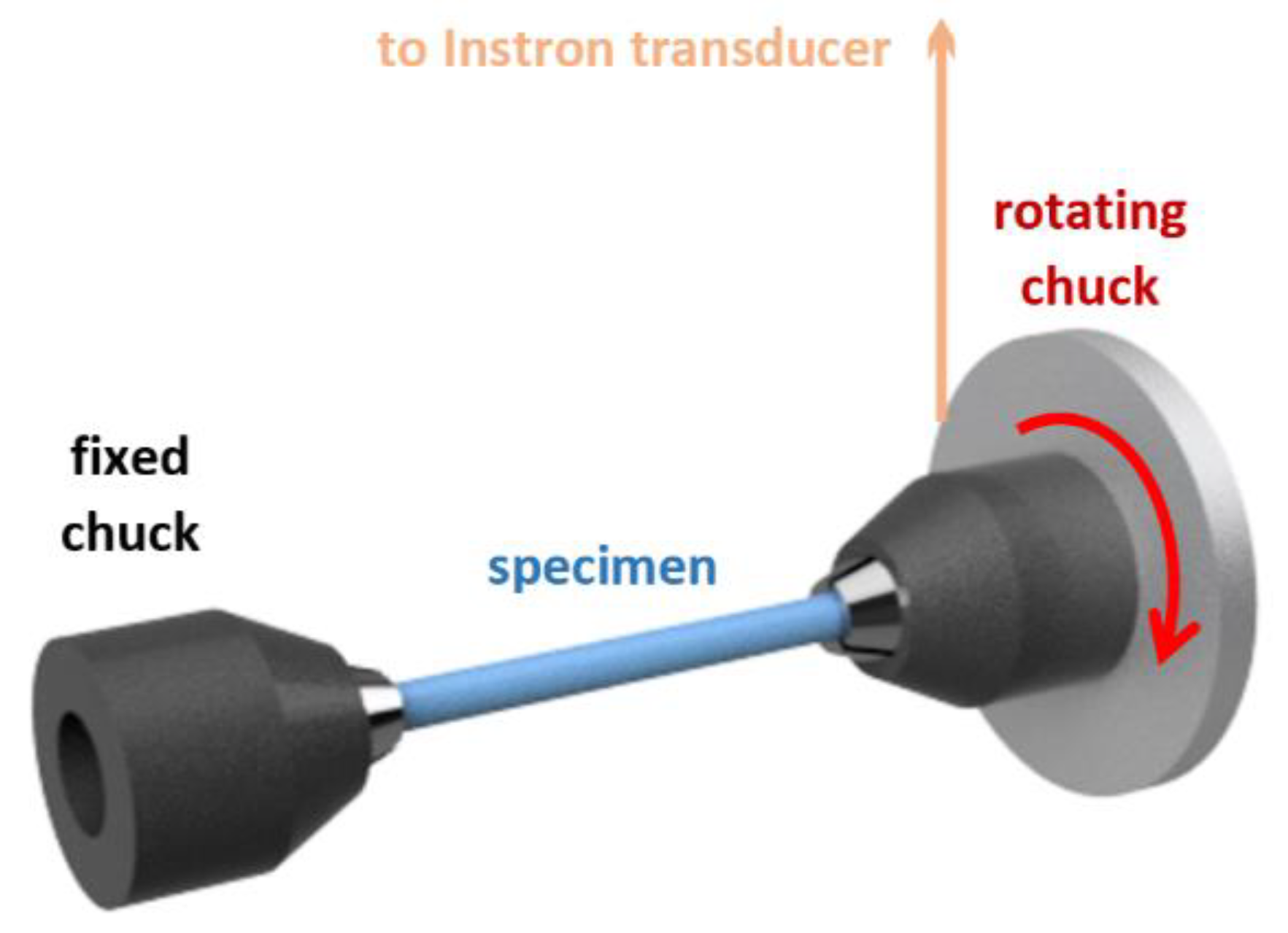
2.6. Mechanical Properties
2.6.1. Young’s Modulus
2.6.2. Tensile Strength
2.6.3. Shear Modulus and Rigidity
2.7. Statistics
3. Results and Discussion
3.1. Stent Degradation
3.2. Thermal Analysis
3.2.1. Thermogravimetric Analysis
3.2.2. Thermal Behavior and Determination of Crystallinity
3.3. Scanning Electron Microscopy
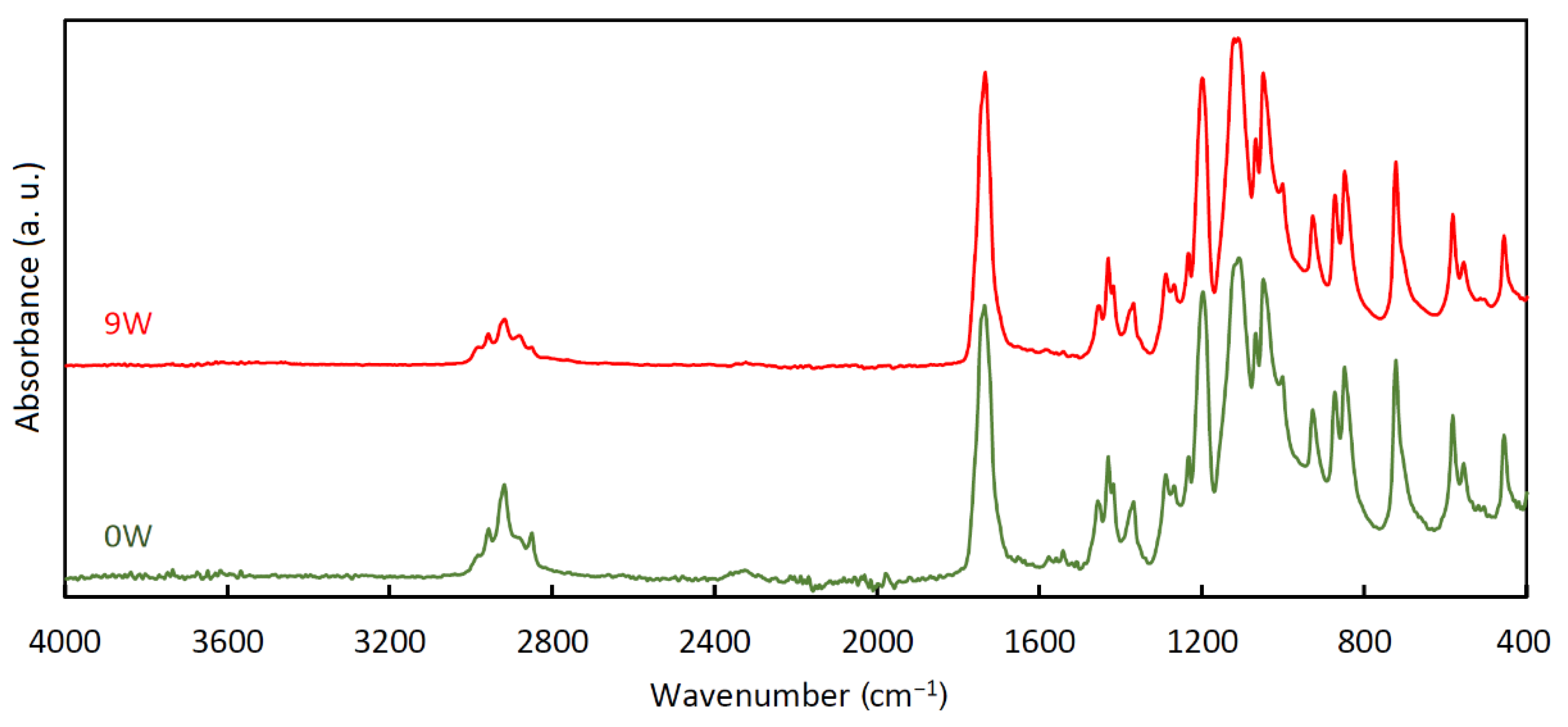
3.4. Infrared Spectroscopy
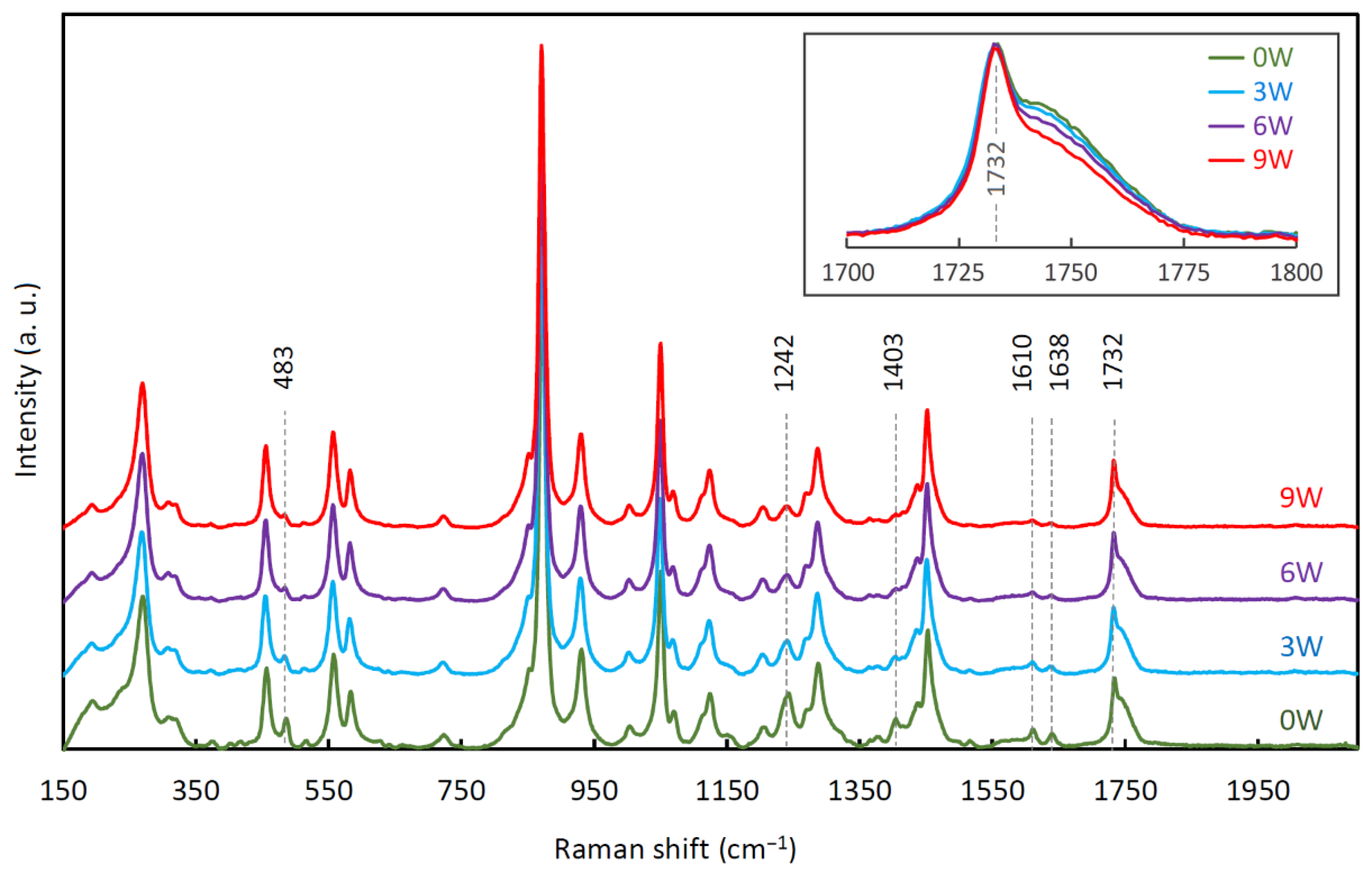
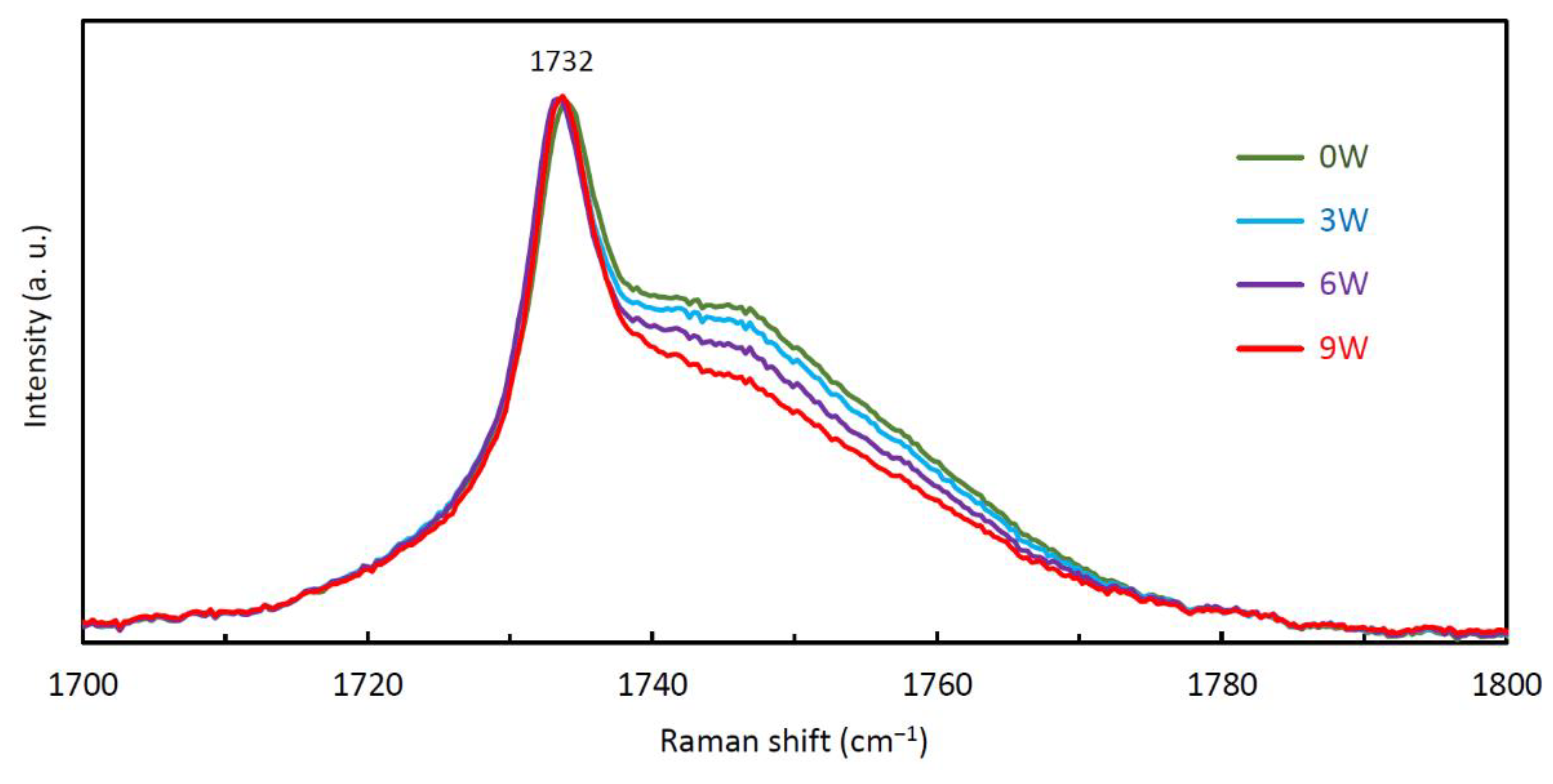
3.5. Raman Spectroscopy
3.6. Mechanical Properties
3.6.1. Young’s Modulus
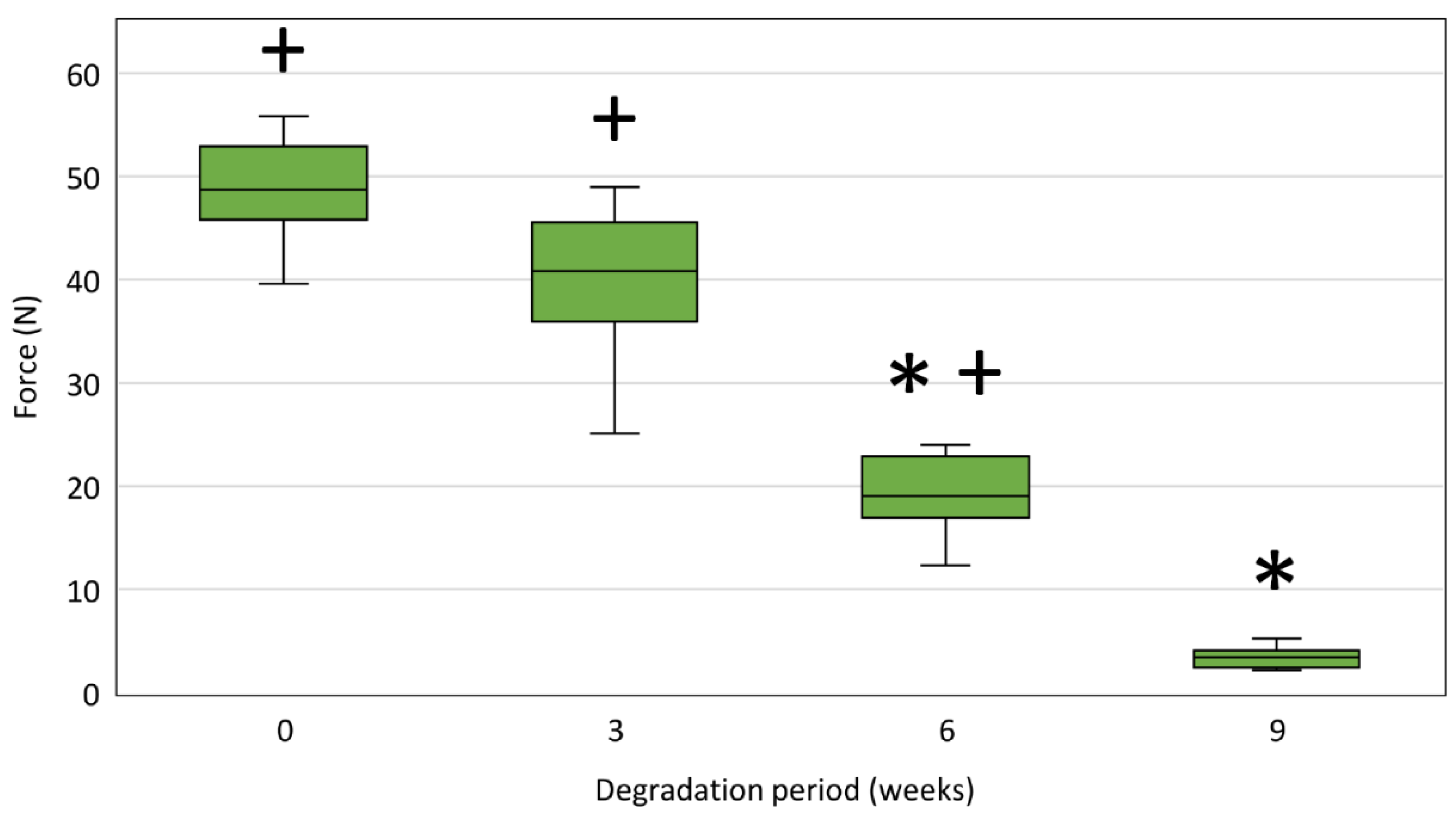
3.6.2. Tensile Strength
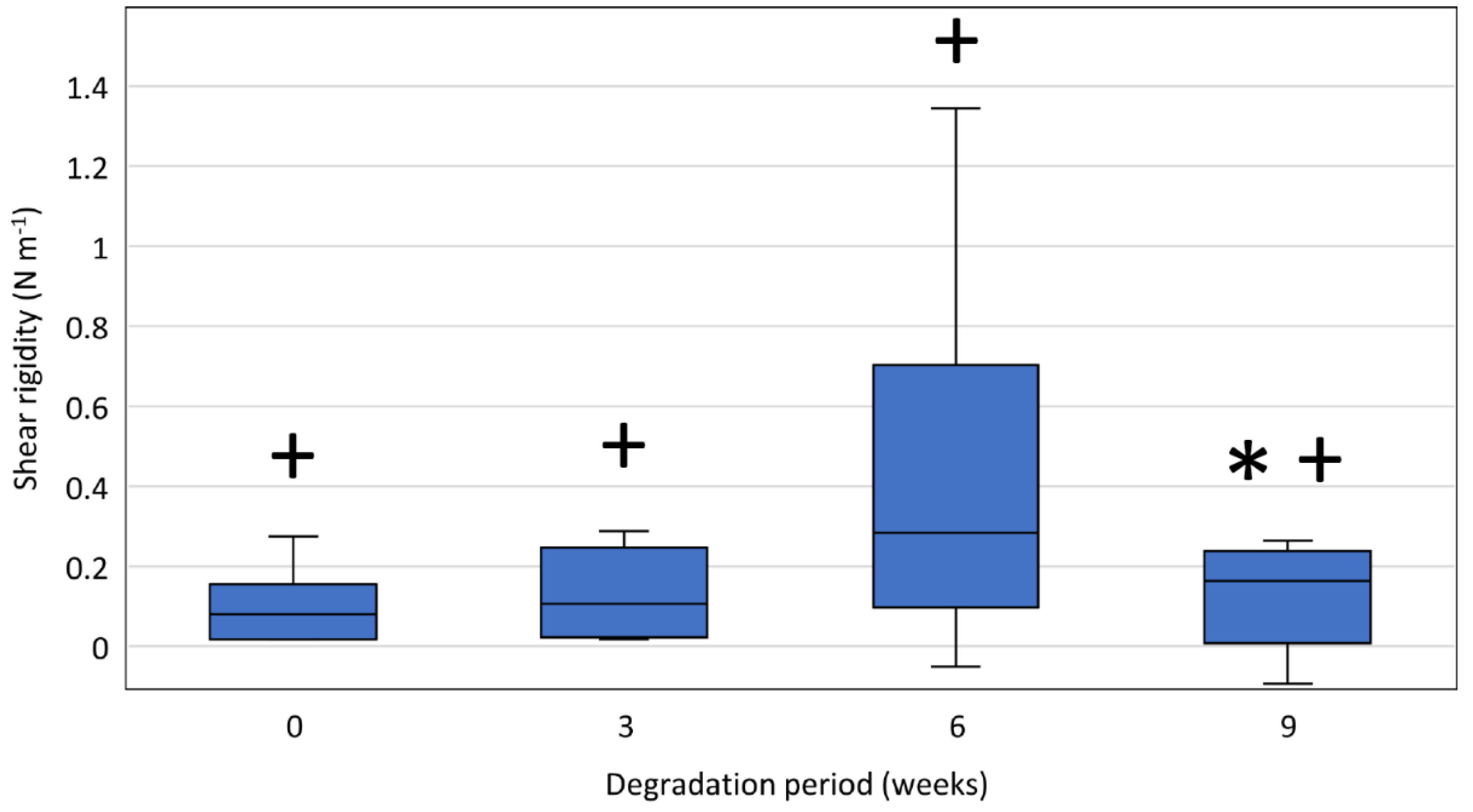
3.6.3. Shear Modulus and Rigidity
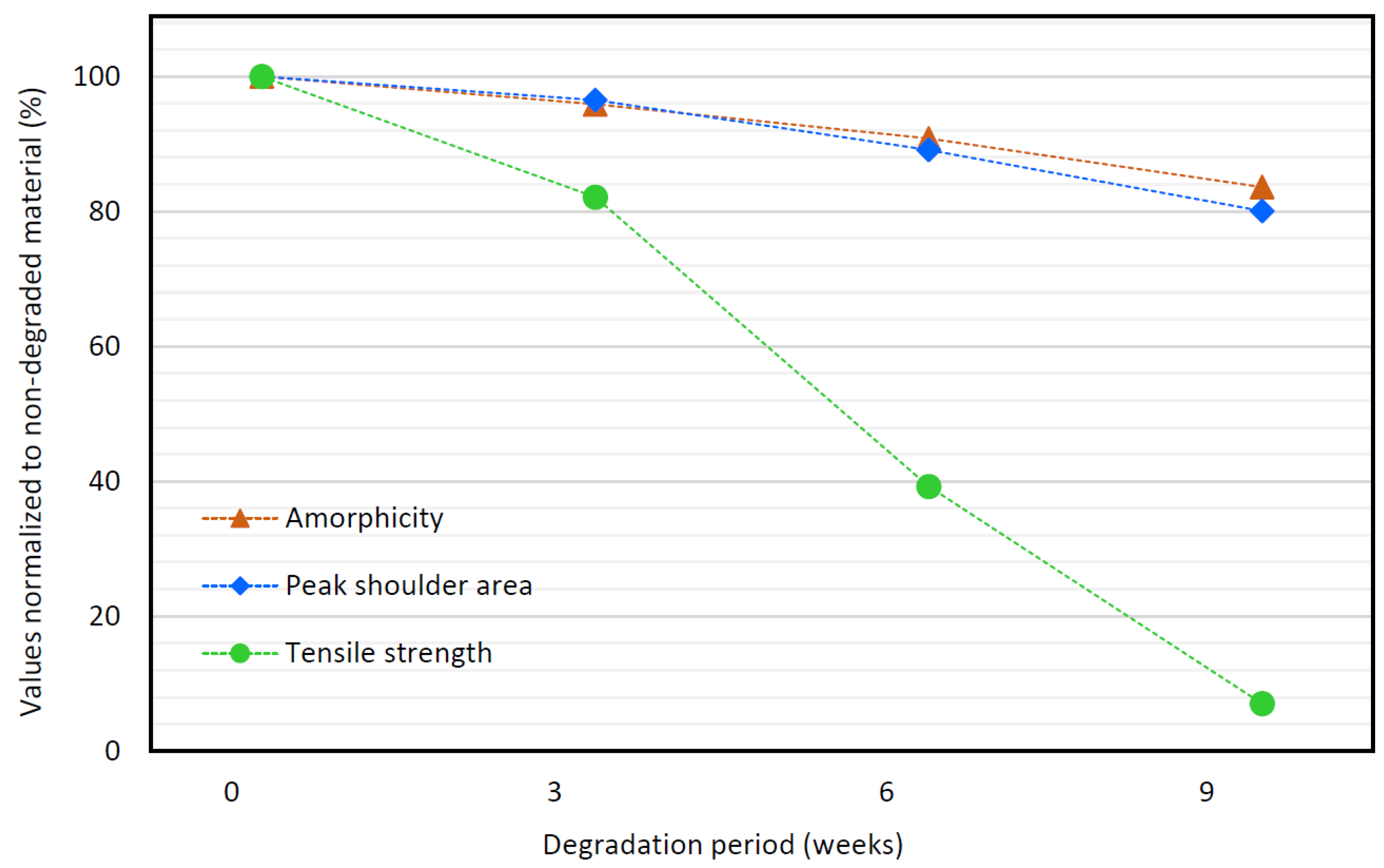
3.7. Comparison of DSC, RS, and Tensile Strength Data
3.8. Strengths and Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Škrlová, K.; Malachová, K.; Muñoz-Bonilla, A.; Měřinská, D.; Rybková, Z.; Fernández-García, M.; Plachá, D. Biocompatible Polymer Materials with Antimicrobial Properties for Preparation of Stents. Nanomaterials 2019, 9, 1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aibibu, D.; Hild, M.; Cherif, C. 6—An overview of braiding structure in medical textile: Fiber-based implants and tissue engineering. In Advances in Braiding Technology, Kyosev, Y., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 171–190. [Google Scholar]
- Mbah, N.; Philips, P.; Voor, M.J.; Martin, R.C.G., 2nd. Optimal radial force and size for palliation in gastroesophageal adenocarcinoma: A comparative analysis of current stent technology. Surg. Endosc. 2017, 31, 5076–5082. [Google Scholar] [CrossRef] [PubMed]
- Hirdes, M.M.; Vleggaar, F.P.; de Beule, M.; Siersema, P.D. In vitro evaluation of the radial and axial force of self-expanding esophageal stents. Endoscopy 2013, 45, 997–1005. [Google Scholar] [CrossRef] [Green Version]
- Hindy, P.; Hong, J.; Lam-Tsai, Y.; Gress, F. A comprehensive review of esophageal stents. Gastroenterol. Hepatol. 2012, 8, 526–534. [Google Scholar]
- Isayama, H.; Mukai, T.; Itoi, T.; Maetani, I.; Nakai, Y.; Kawakami, H.; Yasuda, I.; Maguchi, H.; Ryozawa, S.; Hanada, K.; et al. Comparison of partially covered nitinol stents with partially covered stainless stents as a historical control in a multicenter study of distal malignant biliary obstruction: The WATCH study. Gastrointest. Endosc. 2012, 76, 84–92. [Google Scholar] [CrossRef]
- Ormiston, J.A.; Serruys, P.W. Bioabsorbable coronary stents. Circ. Cardiovasc. Interv. 2009, 2, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Zartner, P.; Buettner, M.; Singer, H.; Sigler, M. First biodegradable metal stent in a child with congenital heart disease: Evaluation of macro and histopathology. Catheter. Cardiovasc. Interv. 2007, 69, 443–446. [Google Scholar] [CrossRef]
- Alexy, R.D.; Levi, D.S. Materials and Manufacturing Technologies Available for Production of a Pediatric Bioabsorbable Stent. BioMed. Res. Int. 2013, 2013, 137985. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Jiang, M.; Gao, Y.; Zheng, Y.; Liu, Z.; Zhou, C.; Huang, T.; Gu, X.; Li, A.; Fang, J.; et al. Current status and outlook of biodegradable metals in neuroscience and their potential applications as cerebral vascular stent materials. Bioact. Mater. 2021, 11, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Petrtýl, J.; Brůha, R.; Horák, L.; Zádorová, Z.; Dosedel, J.; Laasch, H.U. Management of benign intrahepatic bile duct strictures: Initial experience with polydioxanone biodegradable stents. Endoscopy 2010, 42 (Suppl. S2), E89–E90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoi, T.; Kasuya, K.; Abe, Y.; Isayama, H. Endoscopic placement of a new short-term biodegradable pancreatic and biliary stent in an animal model: A preliminary feasibility study (with videos). J. Hepato-Biliary-Pancreat. Sci. 2011, 18, 463–467. [Google Scholar] [CrossRef]
- Cheon, K.-H.; Park, C.; Kang, M.-H.; Park, S.; Kim, J.; Jeong, S.-H.; Kim, H.-E.; Jung, H.-D.; Jang, T.-S. A combination strategy of functionalized polymer coating with Ta ion implantation for multifunctional and biodegradable vascular stents. J. Magnes. Alloy. 2021, 9, 2194–2206. [Google Scholar] [CrossRef]
- Lorenzo-Zúñiga, V.; Moreno-de-Vega, V.; Marín, I.; Boix, J. Biodegradable stents in gastrointestinal endoscopy. World J. Gastroenterol. 2014, 20, 2212–2217. [Google Scholar] [CrossRef]
- Adhikari, K.R.; Stanishevskaya, I.; Caracciolo, P.C.; Abraham, G.A.; Thomas, V. Novel Poly(ester urethane urea)/Polydioxanone Blends: Electrospun Fibrous Meshes and Films. Molecules 2021, 26, 3847. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Ha, D.-H.; Chae, S.; Lee, J.Y.; Kim, J.Y.; Yoon, J.; Sen, T.; Lee, S.-W.; Kim, H.J.; Cho, J.H.; Cho, D.-W. Therapeutic effect of decellularized extracellular matrix-based hydrogel for radiation esophagitis by 3D printed esophageal stent. Biomaterials 2021, 266, 120477. [Google Scholar] [CrossRef]
- Göpferich, A. Mechanisms of polymer degradation and erosion. Biomaterials 1996, 17, 103–114. [Google Scholar] [CrossRef]
- Lalezari, D.; Singh, I.; Reicher, S.; Eysselein, V.E. Evaluation of fully covered self-expanding metal stents in benign biliary strictures and bile leaks. World J. Gastrointest. Endosc. 2013, 5, 332–339. [Google Scholar] [CrossRef] [PubMed]
- García-Cano, J. Endoscopic management of benign biliary strictures. Curr. Gastroenterol. Rep. 2013, 15, 336. [Google Scholar] [CrossRef]
- Gwon, D.I.; Ko, G.Y.; Ko, H.K.; Yoon, H.K.; Sung, K.B. Percutaneous transhepatic treatment using retrievable covered stents in patients with benign biliary strictures: Mid-term outcomes in 68 patients. Dig. Dis. Sci. 2013, 58, 3270–3279. [Google Scholar] [CrossRef] [PubMed]
- Mauri, G.; Michelozzi, C.; Melchiorre, F.; Poretti, D.; Pedicini, V.; Salvetti, M.; Criado, E.; Falcò Fages, J.; De Gregorio, M.; Laborda, A.; et al. Benign biliary strictures refractory to standard bilioplasty treated using polydoxanone biodegradable biliary stents: Retrospective multicentric data analysis on 107 patients. Eur. Radiol. 2016, 26, 4057–4063. [Google Scholar] [CrossRef] [PubMed]
- Siiki, A.; Sand, J.; Laukkarinen, J. A systematic review of biodegradable biliary stents: Promising biocompatibility without stent removal. Eur. J. Gastroenterol. Hepatol. 2018, 30, 813–818. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, M.A.; Criado, E.; Guirola, J.A.; Alvarez-Arranz, E.; Pérez-Lafuente, M.; Barrufet, M.; Ferrer-Puchol, M.D.; Lopez-Minguez, S.; Urbano, J.; Lanciego, C.; et al. Absorbable stents for treatment of benign biliary strictures: Long-term follow-up in the prospective Spanish registry. Eur. Radiol. 2020, 30, 4486–4495. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, M.W.; Walter, D.; de Vries, E.M.; Vleggaar, F.P.; van Berge Henegouwen, M.I.; van Hillegersberg, R.; Siersema, P.D.; Fockens, P.; van Hooft, J.E. Biodegradable stent placement before neoadjuvant chemoradiotherapy as a bridge to surgery in patients with locally advanced esophageal cancer. Gastrointest. Endosc. 2014, 80, 908–913. [Google Scholar] [CrossRef]
- del-Pozo-García, A.J.; Piedracoba-Cadahia, C.; Sánchez-Gómez, F.; Marín-Gabriel, J.C.; Rodríguez-Muñoz, S. Complete resolution of dysphagia after sequential Polyflex (tm) stenting in a case of recurrent anastomotic stenosis in an adult with congenital esophageal atresia. Rev. Española De Enferm. Dig. 2018, 110, 826–829. [Google Scholar]
- Walter, D.; van den Berg, M.W.; Hirdes, M.M.; Vleggaar, F.P.; Repici, A.; Deprez, P.H.; Viedma, B.L.; Lovat, L.B.; Weusten, B.L.; Bisschops, R.; et al. Dilation or biodegradable stent placement for recurrent benign esophageal strictures: A randomized controlled trial. Endoscopy 2018, 50, 1146–1155. [Google Scholar] [CrossRef]
- Nogales, Ó.; Clemente, A.; Caballero-Marcos, A.; García-Lledó, J.; Pérez-Carazo, L.; Merino, B.; López-Ibáñez, M.; Pérez Valderas, M.D.; Bañares, R.; González-Asanza, C. Endoscopically placed stents: A useful alternative for the management of refractory benign cervical esophageal stenosis. Rev. Esp. Enferm. Dig. Organo Soc. Esp. Patol. Dig. 2017, 109, 510–515. [Google Scholar] [CrossRef] [Green Version]
- Hirdes, M.M.; Siersema, P.D.; van Boeckel, P.G.; Vleggaar, F.P. Single and sequential biodegradable stent placement for refractory benign esophageal strictures: A prospective follow-up study. Endoscopy 2012, 44, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Rejchrt, S.; Kopacova, M.; Brozik, J.; Bures, J. Biodegradable stents for the treatment of benign stenoses of the small and large intestines. Endoscopy 2011, 43, 911–917. [Google Scholar] [CrossRef]
- Griffiths, B.T.; James, P.; Morgan, G.; Diamantopoulos, A.; Durward, A.; Nyman, A. Biodegradable Stents for the Relief of Vascular Bronchial Compression in Children With Left Atrial Enlargement. J. Bronchol. Interv. Pulmonol. 2020, 27, 200–204. [Google Scholar] [CrossRef]
- Stehlik, L.; Hytych, V.; Letackova, J.; Kubena, P.; Vasakova, M. Biodegradable polydioxanone stents in the treatment of adult patients with tracheal narrowing. BMC Pulm. Med. 2015, 15, 164. [Google Scholar] [CrossRef] [Green Version]
- Novotny, L.; Crha, M.; Rauser, P.; Hep, A.; Misik, J.; Necas, A.; Vondrys, D. Novel biodegradable polydioxanone stents in a rabbit airway model. J. Thorac. Cardiovasc. Surg. 2012, 143, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Stramiello, J.A.; Mohammadzadeh, A.; Ryan, J.; Brigger, M.T. The role of bioresorbable intraluminal airway stents in pediatric tracheobronchial obstruction: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2020, 139, 110405. [Google Scholar] [CrossRef] [PubMed]
- Antón-Pacheco, J.L.; Luna, C.; García, E.; López, M.; Morante, R.; Tordable, C.; Palacios, A.; de Miguel, M.; Benavent, I.; Gómez, A. Initial experience with a new biodegradable airway stent in children: Is this the stent we were waiting for? Pediatric Pulmonol. 2016, 51, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Siiki, A.; Rinta-Kiikka, I.; Sand, J.; Laukkarinen, J. Endoscopic biodegradable biliary stents in the treatment of benign biliary strictures: First report of clinical use in patients. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2017, 29, 118–121. [Google Scholar] [CrossRef]
- Giménez, M.E.; Palermo, M.; Houghton, E.; Acquafresca, P.; Finger, C.; Verde, J.M.; Cúneo, J.C. Biodegradable biliary stents: A new approach for the management of hepaticojejunostomy strictures following bile duct injury. prospective study. Arq. Bras. De Cir. Dig. ABCD = Braz. Arch. Dig. Surg. 2016, 29, 112–116. [Google Scholar] [CrossRef] [Green Version]
- Battistel, M.; Senzolo, M.; Ferrarese, A.; Lupi, A.; Cillo, U.; Boccagni, P.; Zanus, G.; Stramare, R.; Quaia, E.; Burra, P.; et al. Biodegradable Biliary Stents for Percutaneous Treatment of Post-liver Transplantation Refractory Benign Biliary Anastomotic Strictures. Cardiovasc. Interv. Radiol. 2020, 43, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Rejchrt, S.; Kopáčová, M.; Bártová, J.; Vacek, Z.; Bureš, J. Intestinal biodegradable stents. Folia Gastroenterol. Hepatol. 2009, 7, 7–11. [Google Scholar]
- Doddi, N.; Versfelt, C.C.; Wasserman, D. Synthetic Absorbable Surgical Devices of Poly-Dioxanone. U.S. Patent No. 4,052,988, 11 October 1977. [Google Scholar]
- Ishikiriyama, K.; Pyda, M.; Zhang, G.; Forschner, T.; Grebowicz, J.; Wunderlich, B. Heat capacity of poly-p-dioxanone. J. Macromol. Sci. Part B 1998, 37, 27–44. [Google Scholar] [CrossRef]
- Yang, K.-K.; Wang, X.-L.; Wang, Y.-Z. Poly (p-dioxanone) and its copolymers. J. Macromol. Sci. Part C Polym. Rev. 2002, 42, 373–398. [Google Scholar] [CrossRef]
- Furuhashi, Y.; Nakayama, A.; Monno, T.; Kawahara, Y.; Yamane, H.; Kimura, Y.; Iwata, T. X-Ray and Electron Diffraction Study of Poly (p-dioxanone). Macromol. Rapid Commun. 2004, 25, 1943–1947. [Google Scholar] [CrossRef]
- Gestí, S.; Lotz, B.; Casas, M.T.; Alemán, C.; Puiggali, J. Morphology and structure of poly (p-dioxanone). Eur. Polym. J. 2007, 43, 4662–4674. [Google Scholar] [CrossRef]
- Lin, H.L.; Chu, C.; Grubb, D. Hydrolytic degradation and morphologic study of poly-p-dioxanone. J. Biomed. Mater. Res. 1993, 27, 153–166. [Google Scholar] [CrossRef]
- Ray, J.A.; Doddi, N.; Regula, D.; Williams, J.A.; Melveger, A. Polydioxanone (PDS), a novel monofilament synthetic absorbable suture. Surg. Gynecol. Obstet. 1981, 153, 497–507. [Google Scholar] [PubMed]
- Sabino, M.A.; Feijoo, J.L.; Müller, A.J. Crystallisation and morphology of poly(p-dioxanone). Macromol. Chem. Phys. 2000, 201, 2687–2698. [Google Scholar] [CrossRef]
- Wang, C.-e.; Zhang, P.-h. Design and characterization of PDO biodegradable intravascular stents. Text. Res. J. 2017, 87, 1968–1976. [Google Scholar] [CrossRef]
- Bezrouk, A.; Hosszu, T.; Hromadko, L.; Olmrova Zmrhalova, Z.; Kopecek, M.; Smutny, M.; Selke Krulichova, I.; Macak, J.M.; Kremlacek, J. Mechanical properties of a biodegradable self-expandable polydioxanone monofilament stent: In vitro force relaxation and its clinical relevance. PLoS ONE 2020, 15, e0235842. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, J.; Cheng, J.; Wu, G.; Zhang, Y.; Ni, Z.; Zhao, G. A poly(L-lactic acid) monofilament with high mechanical properties for application in biodegradable biliary stents. J. Appl. Polym. Sci. 2021, 138, 49656. [Google Scholar] [CrossRef]
- Wang, P.-J.; Ferralis, N.; Conway, C.; Grossman, J.C.; Edelman, E.R. Strain-induced accelerated asymmetric spatial degradation of polymeric vascular scaffolds. Proc. Natl. Acad. Sci. USA 2018, 115, 2640–2645. [Google Scholar] [CrossRef] [Green Version]
- Vano-Herrera, K.; Vogt, C. Degradation of poly(l lactic acid) coating on permanent coronary metal stent investigated ex vivo by micro Raman spectroscopy. J. Raman Spectrosc. 2017, 48, 711–719. [Google Scholar] [CrossRef]
- Jaidann, M.; Brisson, J. Conformation study of poly(p-dioxanone) fibers by polarized Raman spectroscopy, X-ray diffraction, and conformation analysis. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 406–417. [Google Scholar] [CrossRef]
- Bower, D.I. An Introduction to Polymer Physics. Am. J. Phys. 2003, 71, 285–286. [Google Scholar] [CrossRef] [Green Version]
- Bower, D.I.; Maddams, W.F. The Vibrational Spectroscopy of Polymers; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Loskot, J.; Jezbera, D.; Bezrouk, A.; Doležal, R.; Andrýs, R.; Francová, V.; Miškář, D.; Myslivcová Fučíková, A. Raman Spectroscopy as a Novel Method for the Characterization of Polydioxanone Medical Stents Biodegradation. Materials 2021, 14, 5462. [Google Scholar] [CrossRef]
- Schrader, B. Vibrational spectroscopy of different classes and states of compounds In Infrared and Raman Spectroscopy—Methods and Applications; Schrader, B., Ed.; VCH: Weinhem, Germany, 1995; p. 190. [Google Scholar]
- Greenwald, D.; Shumway, S.; Albear, P.; Gottlieb, L. Mechanical comparison of 10 suture materials before and after in vivo incubation. J. Surg. Res. 1994, 56, 372–377. [Google Scholar] [CrossRef]
- Kreszinger, M.; Surgery, O.C.f.; Toholj, B.; Banski, A.; Balpa, S.; Cincovic, M.; Pein, M.; Lipar, M.; Smolec, O. Tensile strength retention of resorptive suture materials applied in the stomach wall—An in vitro study. Vet. Arh. 2018, 88, 235–243. [Google Scholar] [CrossRef]
- Ciccone, W.J., II; Motz, C.; Bentley, C.; Tasto, J.P. Bioabsorbable Implants in Orthopaedics: New Developments and Clinical Applications. JAAOS—J. Am. Acad. Orthop. Surg. 2001, 9, 280–288. [Google Scholar] [CrossRef] [Green Version]
- Sabino, M.A.; Albuerne, J.; Müller, A.J.; Brisson, J.; Prud’homme, R.E. Influence of In Vitro Hydrolytic Degradation on the Morphology and Crystallization Behavior of Poly(p-dioxanone). Biomacromolecules 2004, 5, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-F.; Jones, S.; Wang, D.; Wood, A.; Washington, T.; Acreman, K.; Cuevas, B.; Karau, A. Influence of thermal annealing on mechanical properties and in vitro degradation of poly(p-dioxanone). Polym. Eng. Sci. 2019, 59, 1701–1709. [Google Scholar] [CrossRef]
- Chu, C.C. Hydrolytic degradation of polyglycolic acid: Tensile strength and crystallinity study. J. Appl. Polym. Sci. 1981, 26, 1727–1734. [Google Scholar] [CrossRef]
- Välimaa, T.; Laaksovirta, S.; Tammela, T.L.J.; Laippala, P.; Talja, M.; Isotalo, T.; Pétas, A.; Taari, K.; Törmälä, P. Viscoelastic memory and self-expansion of self-reinforced bioabsorbable stents. Biomaterials 2002, 23, 3575–3582. [Google Scholar] [CrossRef]
- Zilberman, M.; Nelson, K.D.; Eberhart, R.C. Mechanical properties and in vitro degradation of bioresorbable fibers and expandable fiber-based stents. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 74B, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Y.; Lan, P.; Li, J.; Zhao, Z.; He, X.; Zhang, J.; Hu, H. Biodegradable weft-knitted intestinal stents: Fabrication and physical changes investigation in vitro degradation. J. Biomed. Mater. Res. Part A 2014, 102, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Černá, M.; Köcher, M.; Válek, V.; Aujeský, R.; Neoral, Č.; Andrašina, T.; Pánek, J.; Mahathmakanthi, S. Covered Biodegradable Stent: New Therapeutic Option for the Management of Esophageal Perforation or Anastomotic Leak. Cardiovasc. Interv. Radiol. 2011, 34, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Repici, A.; Vleggaar, F.P.; Hassan, C.; van Boeckel, P.G.; Romeo, F.; Pagano, N.; Malesci, A.; Siersema, P.D. Efficacy and safety of biodegradable stents for refractory benign esophageal strictures: The BEST (Biodegradable Esophageal Stent) study. Gastrointest. Endosc. 2010, 72, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Hauser, B.; Devreker, T.; Urbain, D.; Reynaert, H. A Biodegradable Esophageal Stent in the Treatment of a Corrosive Esophageal Stenosis in a Child. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H. Biostatistics 104: Correlational analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]




| Degradation Period | pH |
|---|---|
| 0 weeks | 7.45 ± 0.01 |
| 3 weeks | 7.29 ± 0.21 |
| 6 weeks | 7.23 ± 0.25 |
| 9 weeks | 7.19 ± 0.34 |
| Degradation Period | Crystallinity (%) |
|---|---|
| 0 weeks | 46.4 ± 1.2 |
| 3 weeks | 48.6 ± 2.2 |
| 6 weeks | 51.3 ± 1.9 |
| 9 weeks | 55.2 ± 4.3 |
| Degradation Period | Normalized Area (cm−1) | Amorphicity (%) |
|---|---|---|
| 0 weeks | 11.06 | 53.6 ± 1.2 |
| 3 weeks | 10.67 | 51.4 ± 2.2 |
| 6 weeks | 9.86 | 48.7 ± 1.9 |
| 9 weeks | 8.86 | 44.8 ± 4.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loskot, J.; Jezbera, D.; Zmrhalová, Z.O.; Nalezinková, M.; Alferi, D.; Lelkes, K.; Voda, P.; Andrýs, R.; Fučíková, A.M.; Hosszú, T.; et al. A Complex In Vitro Degradation Study on Polydioxanone Biliary Stents during a Clinically Relevant Period with the Focus on Raman Spectroscopy Validation. Polymers 2022, 14, 938. https://doi.org/10.3390/polym14050938
Loskot J, Jezbera D, Zmrhalová ZO, Nalezinková M, Alferi D, Lelkes K, Voda P, Andrýs R, Fučíková AM, Hosszú T, et al. A Complex In Vitro Degradation Study on Polydioxanone Biliary Stents during a Clinically Relevant Period with the Focus on Raman Spectroscopy Validation. Polymers. 2022; 14(5):938. https://doi.org/10.3390/polym14050938
Chicago/Turabian StyleLoskot, Jan, Daniel Jezbera, Zuzana Olmrová Zmrhalová, Martina Nalezinková, Dino Alferi, Krisztina Lelkes, Petr Voda, Rudolf Andrýs, Alena Myslivcová Fučíková, Tomáš Hosszú, and et al. 2022. "A Complex In Vitro Degradation Study on Polydioxanone Biliary Stents during a Clinically Relevant Period with the Focus on Raman Spectroscopy Validation" Polymers 14, no. 5: 938. https://doi.org/10.3390/polym14050938






