Biodegradable Films Prepared from Pulp Lignocellulose Adhesives of Urea Formaldehyde Resin Modified by Biosulfonate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Preperation
2.2.1. Synthesis of BBS-Modified PUF Resin
2.2.2. Preparation of Degradation Film
2.3. Measurement
2.3.1. Properties of Modification UF Resins
2.3.2. Free Formaldehyde Content Measurement
2.4. Sample Characterization Analysis
2.4.1. Fourier-Transform Infrared (FTIR) Spectroscopy
2.4.2. Thermal Stability of Resins
2.4.3. Differential Scanning Calorimetry (DSC) Analysis
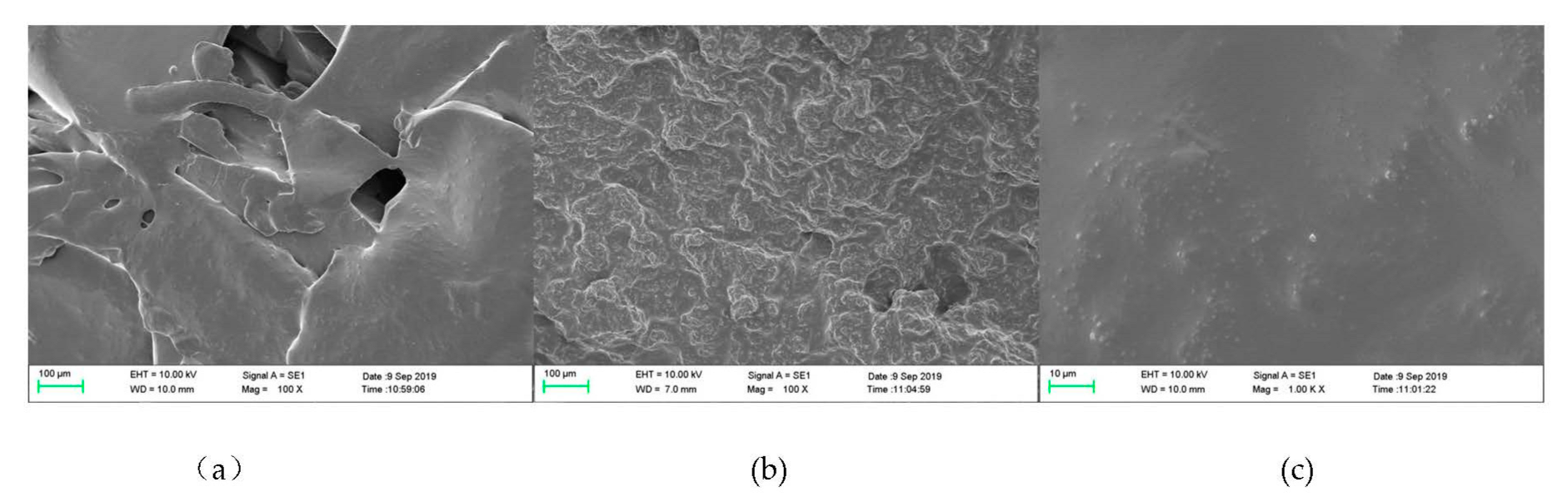
2.4.4. Scanning Electron Microscope (SEM) Analysis
2.5. Degradable Film Performance
2.5.1. Water Retention
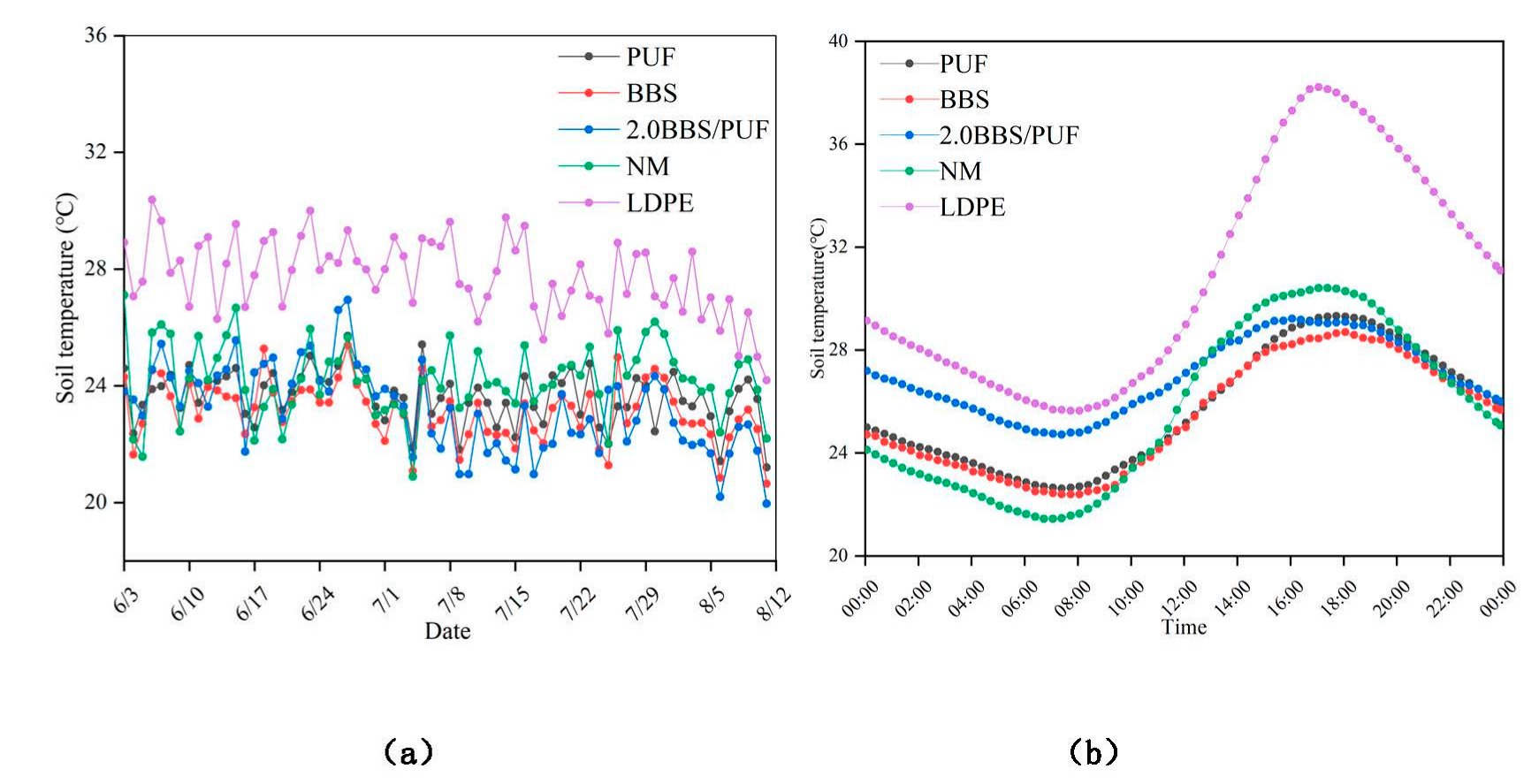
2.5.2. Soil Temperature under Films
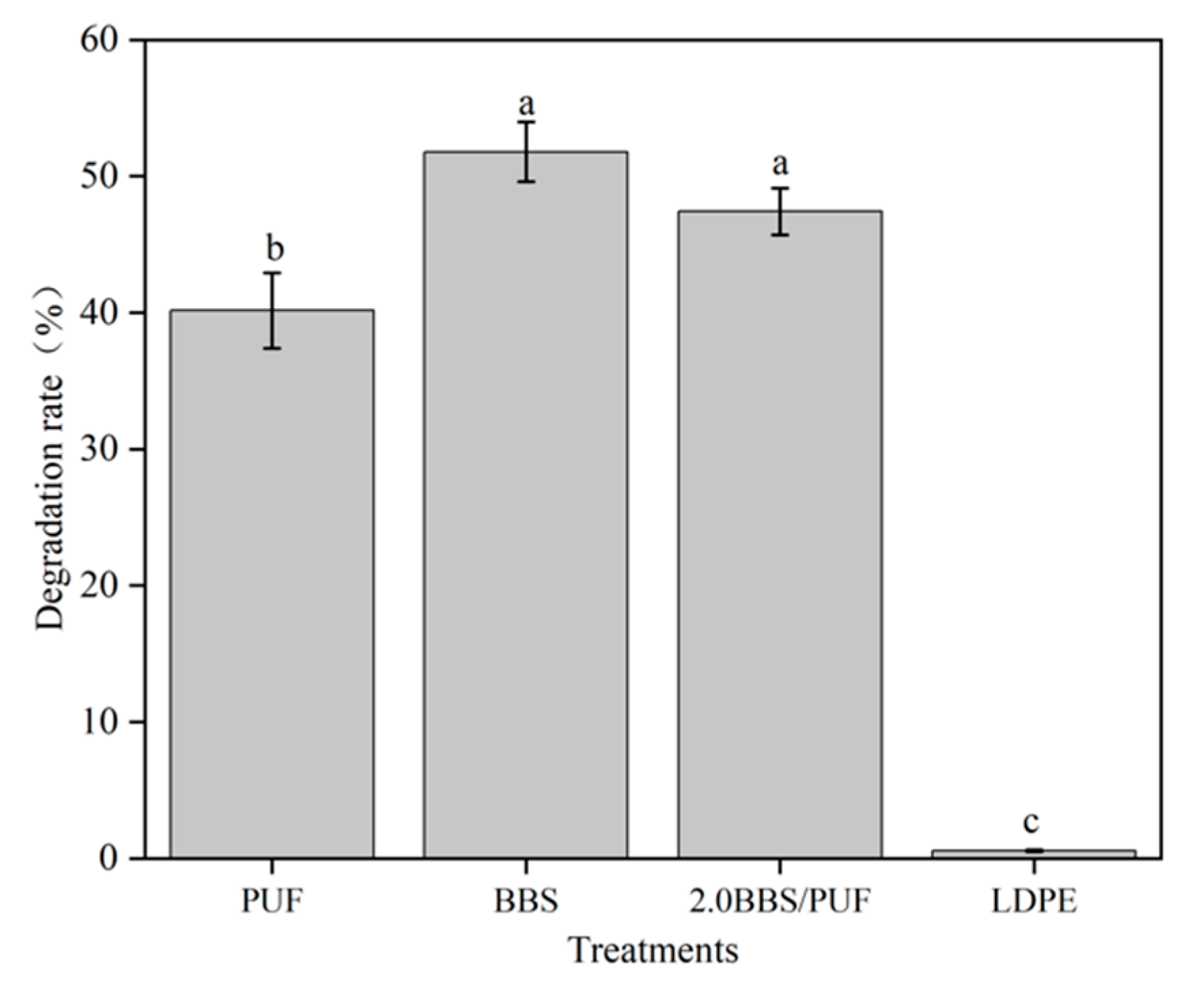
2.5.3. Degradation Rate of Mulch Film
2.6. Statistical Analysis
3. Results
3.1. Physical Properties of Resin
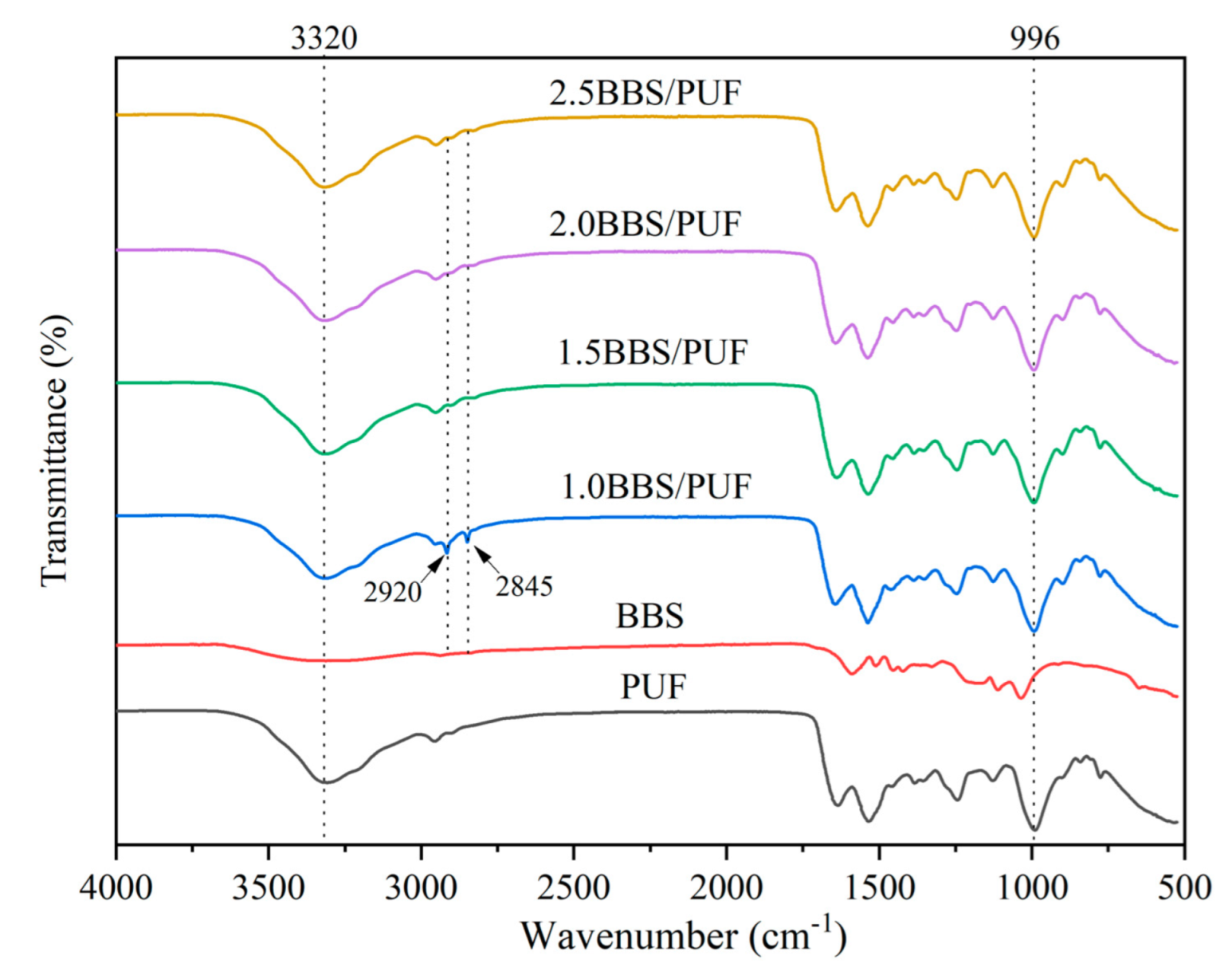
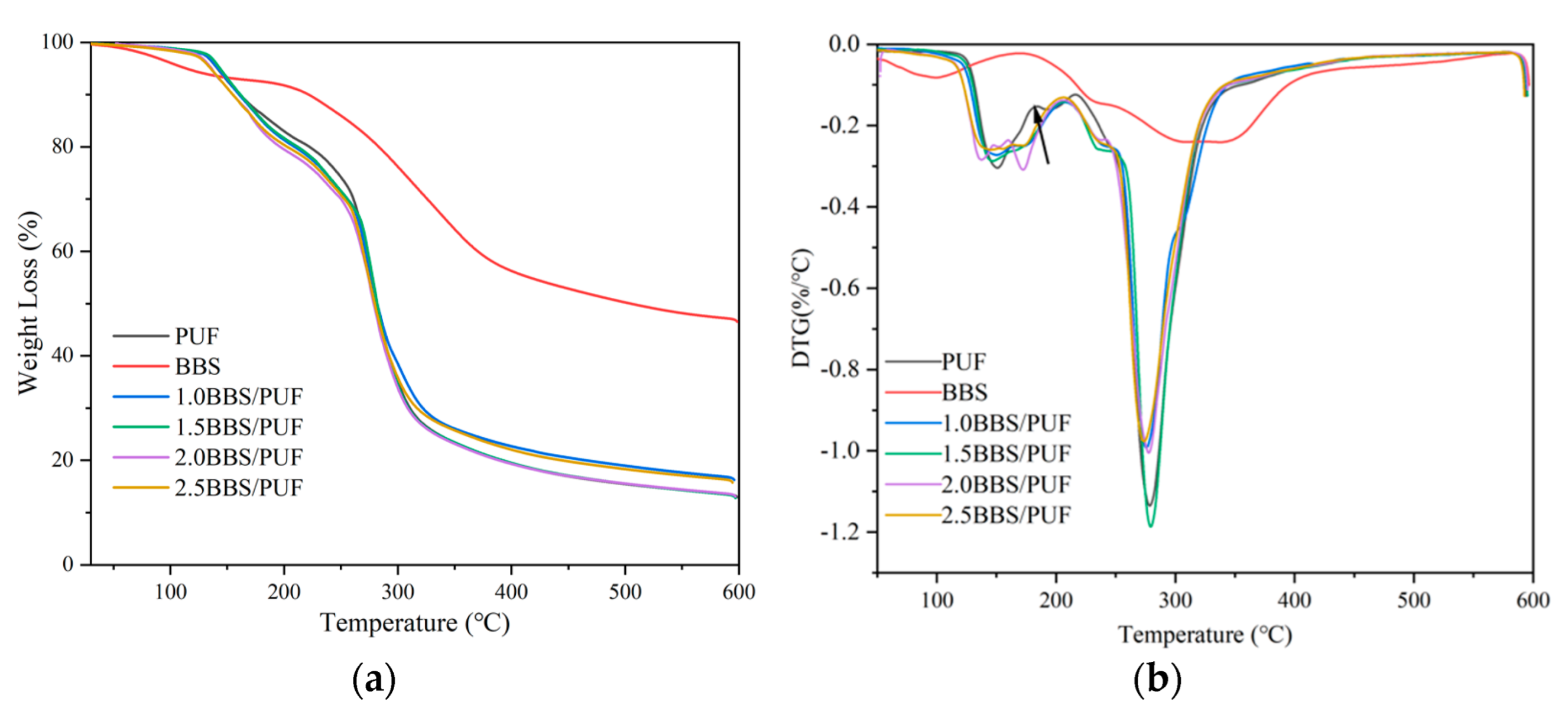
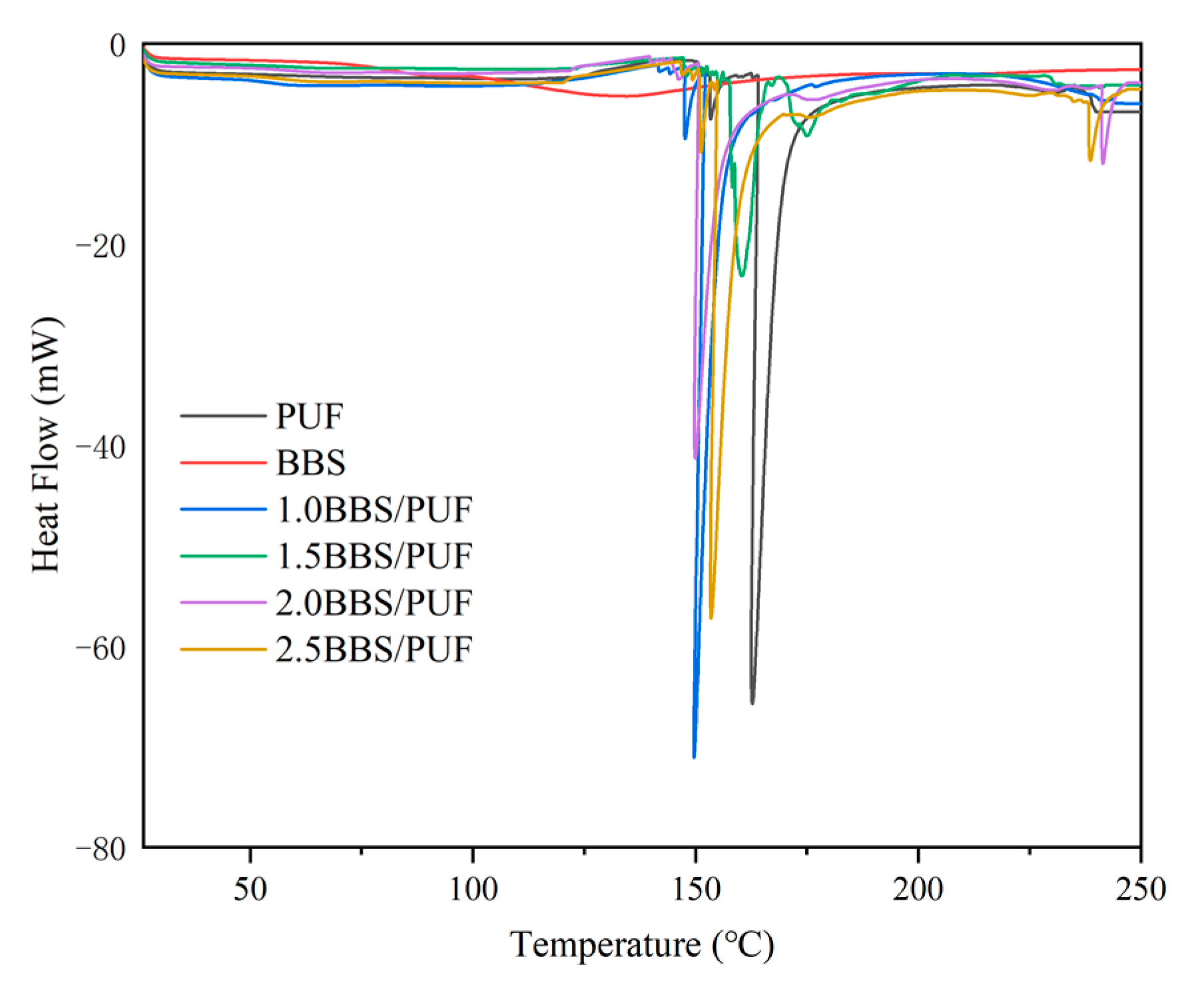
3.2. Characterization Analysis of BBS and PUF Resin
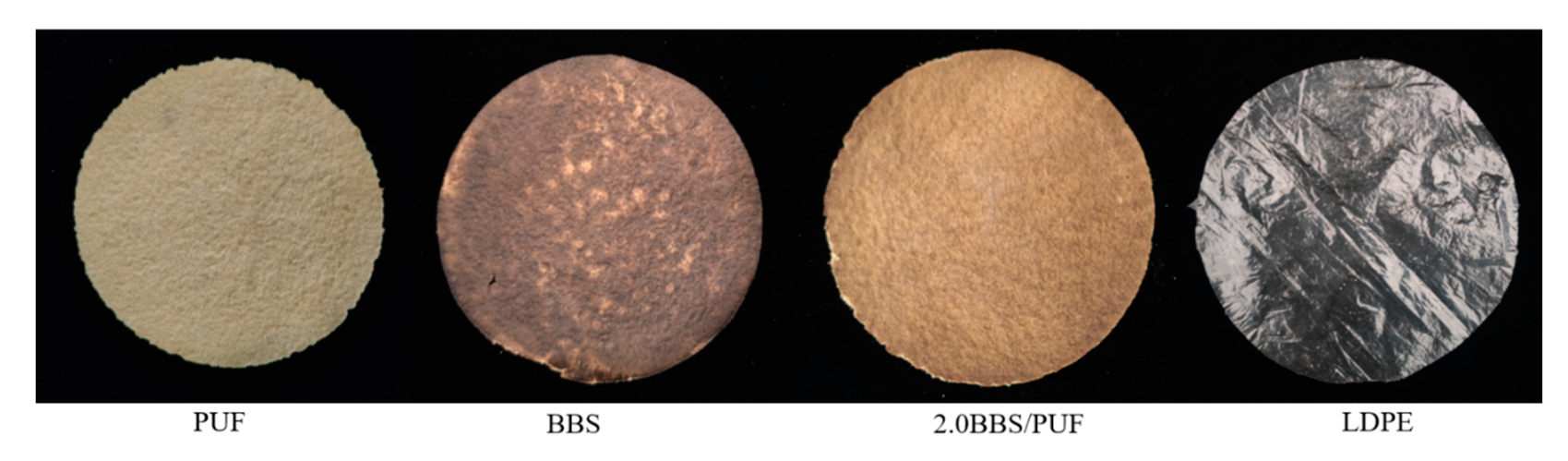
3.3. Analysis of Film Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mo, F.; Wang, J.Y.; Xiong, Y.C.; Nguluu, S.N.; Li, F.-M. Ridge-furrow mulching system in semiarid Kenya: A promising solution to improve soil water availability and maize productivity. Eur. J. Agron. 2016, 80, 124–136. [Google Scholar] [CrossRef]
- Ding, F.; Li, S.; Lü, X.-T.; Dijkstra, F.A.; Schaeffer, S.; An, T.; Pei, J.; Sun, L.; Wang, J. Opposite effects of nitrogen fertilization and plastic film mulching on crop N and P stoichiometry in a temperate agroecosystem. J. Plant Ecol. 2019, 4, 682–692. [Google Scholar] [CrossRef]
- Gao, X.; Xie, D.; Yang, C. Effects of a PLA/PBAT biodegradable film mulch as a replacement of polyethylene film and their residues on crop and soil environment. Agric. Water Manag. 2021, 255, 107053. [Google Scholar] [CrossRef]
- Malinconico, M. Soil Degradable Bioplastics for a Sustainable Modern Agriculture; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Can ridge-furrow plastic mulching replace irrigation in dryland wheat and maize cropping systems? Agric. Water Manag. 2017, 190, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Briassoulis, D.; Babou, E.; Hiskakis, M.; Kyrikou, I. Degradation in soil behavior of artificially aged polyethylene films with pro-oxidants. J. Appl. Polym. Sci. 2015, 132, 3262–3271. [Google Scholar] [CrossRef]
- Ying-Hao, X.; Si-Lin, C.; Zhi-Yu, X.U.; Tuo, J.; Tao, J.; Chang-Rong, Y.; Amp, R.E.; Agency, E.; Agriculture, M.O. Status and trends in application of technology to prevent plastic film residual pollution. J. Agro-Environ. Sci. 2017, 36, 1595–1600. [Google Scholar]
- Gouveia, T.; Biernacki, K.; Castro, M.; Gonalves, M.P.; Souza, H. A new approach to develop biodegradable films based on thermoplastic pectin. Food Hydrocoll. 2019, 97, 105175. [Google Scholar] [CrossRef]
- Zahan, K.A.; Azizul, N.M.; Mustapha, M.; Tong, W.Y.; Rahman, M.S.A.; Sahuri, I.S. Application of bacterial cellulose film as a biodegradable and antimicrobial packaging material. Mater. Today: Proc. 2020, 31, 83–88. [Google Scholar] [CrossRef]
- Zhao, G.; Lyu, X.; Lee, J.; Cui, X.; Chen, W.N. Biodegradable and transparent cellulose film prepared eco-friendly from durian rind for packaging application. Food Packag. Shelf Life 2019, 21, 100345. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Bary, A.I.; Hayes, D.G.; Wadsworth, L.C.; Flury, M. In situ degradation of biodegradable plastic mulch films in compost and agricultural soils. Sci. Total Environ. 2020, 727, 138668. [Google Scholar] [CrossRef]
- Zarandona, I.; Barba, C.; Guerrero, P.; Caba, K.; Maté, J. Development of chitosan films containing β-cyclodextrin inclusion complex for controlled release of bioactives. Food Hydrocoll. 2020, 104, 105720. [Google Scholar] [CrossRef]
- Ar, A.; Cl, A.; Hao, L.A.; Zd, A.; Mn, A.; Mmh, B.; Cl, A.; Js, A.; Dl, A. Chitosan-based biodegradable active food packaging film containing Chinese chive ( Allium tuberosum ) root extract for food application. Int. J. Biol. Macromol. 2020, 150, 595–604. [Google Scholar]
- Maniglia, B.C.; Tessaro, L.; Lucas, A.A.; Tapia-Blacido, D.R. Bioactive films based on babassu mesocarp flour and starch. Food Hydrocoll. 2017, 70, 383–391. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; Oliveira, R.D. Incorporation of spray dried and freeze dried blackberry particles in edible films: Morphology, stability to pH, sterilization and biodegradation. Food Packag. Shelf Life 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.L.; Wang, R.M. Effect of Deinking Process on Wastepaper Fiber Properties. Trans. China Pulp Pap. 2012, 27, 45–49. [Google Scholar]
- Chen, C.; Yihe, Z.; Wanjia, H.; Cheng, Q.; Yongfan, L.; Na, Z. Incorporation of Xuan-paper waste residue in red mud/waste polyethylene composites. J. Hazard. Mater. 2020, 399, 123051. [Google Scholar] [CrossRef]
- Alda, J. Feasibility of recycling pulp and paper mill sludge in the paper and board industries. Resour. Conserv. Recycl. 2008, 52, 965–972. [Google Scholar] [CrossRef]
- Faisal, A.; Salmah, H. Mechanical and Thermal Properties of Compatibilized Waste Office White Paper-Filled Low-Density Polyethylene Composites. J. Thermoplast. Compos. Mater. 2012, 25, 193–207. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, B.; Zhou, X.; Li, L.; Du, G. Influence of Single/Collective Use of Curing Agents on the Curing Behavior and Bond Strength of Soy Protein-Melamine-Urea-Formaldehyde (SMUF) Resin for Plywood Assembly. Polymers 2019, 11, 1995. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Zhang, J.; Gao, Q.; Mao, A.; Li, J. Toughening and Enhancing Melamine–Urea–Formaldehyde Resin Properties via in situ Polymerization of Dialdehyde Starch and Microphase Separation. Polymers 2019, 11, 1167. [Google Scholar] [CrossRef] [Green Version]
- Bekhta, P.; Sedliacik, J.; Kacik, F.; Noshchenko, G.; Kleinova, A. Lignocellulosic waste fibers and their application as a component of urea-formaldehyde adhesive composition in the manufacture of plywood. Eur. J. Wood Wood Prod. 2019, 77, 495–508. [Google Scholar] [CrossRef]
- Pizzi, A.; Papadopoulos, A.N.; Policardi, F. Wood Composites and Their Polymer Binders. Polymers 2020, 12, 1115. [Google Scholar] [CrossRef]
- Mantanis, G.I.; Athanassiadou, E.T.; Barbu, M.C.; Wijnendaele, K. Adhesive systems used in the European particleboard, MDF and OSB industries. Wood Mater. Sci. Eng. 2017, 13, 104–116. [Google Scholar] [CrossRef]
- Chen, S.; Lu, X.; Wang, T.; Zhang, Z. Preparation and characterization of urea-formaldehyde resin/reactive kaolinite composites. Particuology 2016, 32, 783–790. [Google Scholar] [CrossRef]
- Paiva, N.T.; Ferra, J.M.; Pereira, J.; Martins, J.; Carvalho, L.; Magalhães, F.D. Production of water tolerant melamine–urea–formaldehyde resin by incorporation of sodium metabisulphite. Int. J. Adhes. Adhes. 2016, 70, 160–166. [Google Scholar] [CrossRef]
- Kristak, L.; Antov, P.; Bekhta, P.; Lubis, M.A.R.; Iswanto, A.H.; Reh, R.; Sedliacik, J.; Savov, V.; Taghiyari, H.R.; Papadopoulos, A.N.; et al. Recent progress in ultra-low formaldehyde emitting adhesive systems and formaldehyde scavengers in wood-based panels: A review. Wood Mater. Sci. Eng. 2022, 1–20. [Google Scholar] [CrossRef]
- Li, X.; Gao, Q.; Xia, C.; Li, J.; Zhou, X. Urea Formaldehyde Resin Resultant Plywood with Rapid Formaldehyde Release Modified by Tunnel-Structured Sepiolite. Polymers 2019, 11, 1286. [Google Scholar] [CrossRef] [Green Version]
- Srk, A.; Shm, B. Development of active, water-resistant carboxymethyl cellulose-poly vinyl alcohol- Aloe vera packaging film. Carbohydr. Polym. 2020, 227, 115303. [Google Scholar]
- Pan, Y.; Wang, W.; Chao, P.; Kai, S.; Ji, X. Novel hydrophobic polyvinyl alcohol–formaldehyde foams for organic solvents absorption and effective separation. RSC Adv. 2013, 4, 660–669. [Google Scholar] [CrossRef]
- Shen, Y.; Gu, J.; Tan, H.; Lv, S.; Zhang, Y. Preparation and properties of a polyvinyl alcohol toughened urea-formaldehyde foam for thermal insulation applications. Constr. Build. Mater. 2016, 120, 104–111. [Google Scholar] [CrossRef]
- Gungor, F.S.; Kiskan, B. Tailoring polyvinyl alcohol with triazinanes and formaldehyde. React. Funct. Polym. 2018, 124, 115–120. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Jian, Z.; Lu, M. Crystallization behavior of stable urea formaldehyde resin dispersed by polyvinyl alcohol. Iran. Polym. J. 2015, 24, 13–20. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Gassara, F.; Brar, S.K. Biotechnological Applications of Lignin-“Sustainable Alternative to Non-Renewable Materials”. In Lignin: Properties and Applications in Biotechnology and Bio-Energy; Nova Science Pub Inc.: Hauppauge, NY, USA, 2012; ISBN 978-1-61122-907-3. [Google Scholar]
- Ding, C.; Matharu, A.S. Recent Developments on Biobased Curing Agents: A Review of Their Preparation and Use. Acs Sustain. Chem. 2014, 2, 2217–2236. [Google Scholar] [CrossRef]
- Antov, P.; Savov, V.; Mantanis, G.I.; Neykov, N. Medium-density fibreboards bonded with phenol- formaldehyde resin and calcium lignosulfonate as an eco-friendly additive. Wood Mater. Sci. Eng. 2020, 16, 46–48. [Google Scholar] [CrossRef]
- Alonso, M.V.; Oliet, M.; Rodríguez, F.; Astarloa, G.; Echeverría, J. Use of a methylolated softwood ammonium lignosulfonate as partial substitute of phenol in resol resins manufacture. J. Appl. Polym. Sci. 2010, 94, 644–650. [Google Scholar] [CrossRef]
- Antov, P.; Savov, V.; Trichkov, N.; Krišťák, Ľ.; Réh, R.; Papadopoulos, A.N.; Taghiyari, H.R.; Pizzi, A.; Kunecová, D.; Pachikova, M. Properties of High-Density Fiberboard Bonded with Urea–Formaldehyde Resin and Ammonium Lignosulfonate as a Bio-Based Additive. Polymers 2021, 13, 2775. [Google Scholar]
- Bekhta, P.; Noshchenko, G.; Réh, R.; Kristak, L.; Sedliačik, J.; Antov, P.; Mirski, R.; Savov, V. Properties of Eco-Friendly Particleboards Bonded with Lignosulfonate-Urea-Formaldehyde Adhesives and pMDI as a Crosslinker. Materials 2021, 14, 4875. [Google Scholar]
- Mansouri, N.; Salvadó, J. Structural characterization of technical lignins for the production of adhesives: Application to lignosulfonate, kraft, soda-anthraquinone, organosolv and ethanol process lignins. Ind. Crops Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Wang, C.; Chu, F.; Xu, F.; Zhang, D. Synthesis of Lignin-Based Polyacid Catalyst and Its Utilization to Improve Water Resistance of Urea–formaldehyde Resins. Polymers 2020, 12, 175. [Google Scholar] [CrossRef] [Green Version]
- GB/T 14074-2006; Wood Adhesives and Their Resin Test Methods. State Forestry Administration: Beijing, China, 2006.
- Hou, L.; Xi, J.; Chen, X.; Li, X.; Ma, W.; Lu, J.; Xu, J.; Lin, Y.B. Biodegradability and ecological impacts of polyethylene-based mulching film at agricultural environment. J. Hazard. Mater. 2019, 378, 120771–120774. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F. New Synthesis Technology of Biosulfonate/Sulfate and Development of Green Water Reducing Agent; Southwest University: Chongqing, China, 2015. [Google Scholar]
- Alonso, M.V.; Rodríguez, J.J.; Oliet, M.; Rodríguez, F.; García, J.; Gilarranz, M.A. Characterization and structural modification of ammonic lignosulfonate by methylolation. J. Appl. Polym. Sci. 2001, 82, 2661–2668. [Google Scholar] [CrossRef]
- Hu, J.P.; Guo, M.H. Influence of ammonium lignosulfonate on the mechanical and dimensional properties of wood fiber biocomposites reinforced with polylactic acid. Ind. Crops Prod. 2015, 78, 48–57. [Google Scholar] [CrossRef]
- Antov, P.; Savov, V.; Kriák, U.; Réh, R.; Mantanis, G.I. Eco-Friendly, High-Density Fiberboards Bonded with Urea-Formaldehyde and Ammonium Lignosulfonate. Polymers 2021, 13, 220. [Google Scholar] [CrossRef]
- Antov, P.; Mantanis, G.I.; Savov, V. Development of Wood Composites from Recycled Fibres Bonded with Magnesium Lignosulfonate. Forests 2020, 11, 613. [Google Scholar] [CrossRef]
- Antov, P.; Savov, V.; Neykov, N. Sustainable bio-based adhesives for eco-friendly wood composites a review. Wood Res. 2020, 65, 51–62. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.; Dam, J. Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Mansouri, N.E.; Yuan, Q.; Huang, F. Study of chemical modification of alkaline lignin by the glyoxalation reaction. Bioresources 2011, 6, 4523–4536. [Google Scholar]
- Vázquez, G.; González, J.; Freire, S.; Antorrena, G. Effect of chemical modification of lignin on the gluebond performance of lignin-phenolic resins. Bioresour. Technol. 1997, 60, 191–198. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.X.; Sun, R.C.; Fowler, P.; Baird, M.S. Comparative study of organosolv lignins from wheat straw. Ind. Crops Prod. 2006, 23, 180–193. [Google Scholar] [CrossRef]
- Nuryawan, A.; Singh, A.P.; Zanetti, M.; Park, B.; Causin, V. Insights into the development of crystallinity in liquid urea-formaldehyde resins. Int. J. Adhes. Adhes. 2017, 72, 62–69. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y. Morphology and Crystallinity of Urea-Formaldehyde Resin Adhesives with Different Molar Ratios. Polymers 2021, 13, 673. [Google Scholar] [CrossRef] [PubMed]
- Chupin, L.; Charrier, B.; Pizzi, A.; Perdomo, A.; Bouhtoury, C.E. Study of thermal durability properties of tannin-lignosulfonate adhesives. J. Therm. Anal. Calorim. 2015, 119, 1577–1585. [Google Scholar] [CrossRef]
- Alabduljabbar, H.; Alyousef, R.; Gul, W.; Riaz, S.; Alaskar, A. Effect of Alumina Nano-Particles on Physical and Mechanical Properties of Medium Density Fiberboard. Materials 2020, 13, 4207. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Azizli, K.; Man, Z.B.; Ismail, M. Synthesis and Characterization of Urea-formaldehyde Microcapsules Containing Functionalized Polydimethylsiloxanes. Procedia Eng. 2016, 148, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Gan, W. Effect of Copper (II) Sulfate on the Properties of Urea Formaldehyde Adhesive. Polymers 2021, 14, 94. [Google Scholar]
- Lin, S.; Du, S.; Lu, Y. Preparation and Characterization of Submicron TiO2 Microspheres via Urea-formaldehyde Template. IOP Conf. Ser. Mater. Sci. Eng. 2020, 735, 012004–012005. [Google Scholar] [CrossRef]
- Hemmilä, V.; Hosseinpourpia, R.; Adamopoulos, S.; Eceiza, A. Characterization of Wood-based Industrial Biorefinery Lignosulfonates and Supercritical Water Hydrolysis Lignin. Waste Biomass Valorization 2020, 11, 5835–5845. [Google Scholar] [CrossRef] [Green Version]
- Cademartori, P.; Artner, M.; Freitas, R.; Magalhães, W. Alumina nanoparticles as formaldehyde scavenger for urea-formaldehyde resin: Rheological and in-situ cure performance. Compos. Part B: Eng. 2019, 176, 107281. [Google Scholar] [CrossRef]
- Hui, W.; Jiankun, L.; Jun, Z.; Xiaojian, Z.; Guanben, D. Performance of Urea-Formaldehyde Adhesive with Oxidized Cassava Starch. BIORESOURCES 2017, 12, 7590–7600. [Google Scholar]
- Li, P.; Wu, Y.; Liu, W.; Zuo, Y.; Jianxiong, L.; Tu, R. Preparation of Resorcinol-Dialdehyde Starch-Formaldehyde Copolycondensation Resin Adhesive. Gaofenzi Cailiao Kexue Yu Gongcheng/Polym. Mater. Sci. Eng. 2019, 35, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Hemmilä, V.; Adamopoulos, S.; Hosseinpourpia, R.; Ahmed, S.A. Ammonium Lignosulfonate Adhesives for Particleboards with pMDI and Furfuryl Alcohol as Crosslinkers. Polymers 2019, 11, 1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez, J.; Grivel, J.C.; Madsen, B. Study on the non-isothermal curing kinetics of a polyfurfuryl alcohol bioresin by DSC using different amounts of catalyst. Thermochim. Acta 2012, 529, 29–35. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Zhou, J.; Zhu, K.; Sun, S. Degradability of biodegradable plastic films and its mulching effects on soil temperature and maize yield in northeastern China. Int. J. Agric. Biol. Eng. 2020, 13, 146–153. [Google Scholar] [CrossRef]
- Gu, X.-B.; Li, Y.-N.; Du, Y.-D. Biodegradable film mulching improves soil temperature, moisture and seed yield of winter oilseed rape (Brassica napus L.). Soil Tillage Res. 2017, 171, 42–50. [Google Scholar] [CrossRef]
- Zhao, S.; Zuo, H.; Wei, X. Numerical simulation of climate effect in East Asia by plastic film mulching farmland in arid area. Arid Zone Res. 2018, 35, 1363–1372. [Google Scholar]
- Kader, M.A.; Nakamura, K.; Senge, M.; Mojid, M.A.; Kawashima, S. Effects of coloured plastic mulch on soil hydrothermal characteristics, growth and water productivity of rainfed soybean. Irrig. Drain. 2020, 69, 483–494. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Ihl, M.; Sobral, P.d.A.; Gómez-Guillén, M.; Bifani, V. Natural additives in bioactive edible films and coatings: Functionality and applications in foods. Food Eng. Rev. 2013, 5, 200–216. [Google Scholar] [CrossRef]
- Dang, X.; Shan, Z.; Chen, H. The preparation and applications of one biodegradable liquid film mulching by oxidized corn starch-gelatin composite. Appl. Biochem. Biotechnol. 2016, 180, 917–929. [Google Scholar] [CrossRef]
- Bhagat, P.; Gosal, S.; Singh, C. Effect of mulching on soil environment, microbial flora and growth of potato under field conditions. Indian J. Agric. Res. 2016, 50, 542–548. [Google Scholar]
- Wang, Y.; Jia, X.; Olasupo, I.O.; Feng, Q.; Wang, L.; Lu, L.; Xu, J.; Sun, M.; Yu, X.; Han, D. Effects of biodegradable films on melon quality and substrate environment in solar greenhouse. Sci. Total Environ. 2022, 829, 154527. [Google Scholar] [CrossRef] [PubMed]

| Component | C% | H% | N% | S% | C/N | H/C | C/S |
|---|---|---|---|---|---|---|---|
| Biosulfonate A | 24.88 | 3.57 | 0.32 | 10.51 | 78.14 | 1.72 | 6.31 |
| Biosulfonate B | 25.08 | 4.01 | 0.51 | 10.74 | 49.19 | 1.92 | 6.23 |
| Resin | pH | Viscosity (mPa·s) | Free Formaldehyde (%) | Solid Content (%) | Curing Time (s) |
|---|---|---|---|---|---|
| PUF | 7.2 ± 0.10 | 297.97 ± 0.68 | 0.54 ± 0.01 | 52.95 ± 0.106 | 65 ± 1 |
| 1.0BBS/PUF | 7.86 ± 0.08 | 432.33 ± 2.43 | 0.41 ± 0.01 | 54.59 ± 0.212 | 75 ± 1 |
| 1.5BBS/PUF | 8.49 ± 0.12 | 2343.63 ± 3.38 | 0.35 ± 0.01 | 53.41 ± 0.179 | 79 ± 1 |
| 2.0BBS/PUF | 8.25 ± 0.11 | 3319.87 ± 13.97 | 0.27 ± 0.01 | 53.86 ± 0.111 | 81 ± 1 |
| 2.5BBS/PUF | 8.57 ± 0.09 | 4043.20 ± 38.79 | 0.26 ± 0.01 | 53.15 ± 0.171 | 84 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Luo, Y.; Zhang, Q.; Gao, Y.; Li, J.; Shah, S.; Wang, X.; Zhang, X. Biodegradable Films Prepared from Pulp Lignocellulose Adhesives of Urea Formaldehyde Resin Modified by Biosulfonate. Polymers 2022, 14, 2863. https://doi.org/10.3390/polym14142863
Ma Y, Luo Y, Zhang Q, Gao Y, Li J, Shah S, Wang X, Zhang X. Biodegradable Films Prepared from Pulp Lignocellulose Adhesives of Urea Formaldehyde Resin Modified by Biosulfonate. Polymers. 2022; 14(14):2863. https://doi.org/10.3390/polym14142863
Chicago/Turabian StyleMa, Yongjie, Yanxin Luo, Qiannan Zhang, Yanming Gao, Jianshe Li, Sadiq Shah, Xiaozhuo Wang, and Xueyan Zhang. 2022. "Biodegradable Films Prepared from Pulp Lignocellulose Adhesives of Urea Formaldehyde Resin Modified by Biosulfonate" Polymers 14, no. 14: 2863. https://doi.org/10.3390/polym14142863
APA StyleMa, Y., Luo, Y., Zhang, Q., Gao, Y., Li, J., Shah, S., Wang, X., & Zhang, X. (2022). Biodegradable Films Prepared from Pulp Lignocellulose Adhesives of Urea Formaldehyde Resin Modified by Biosulfonate. Polymers, 14(14), 2863. https://doi.org/10.3390/polym14142863





