Evaluation of Antiviral Activity of Cyclic Ketones against Mayaro Virus
Abstract
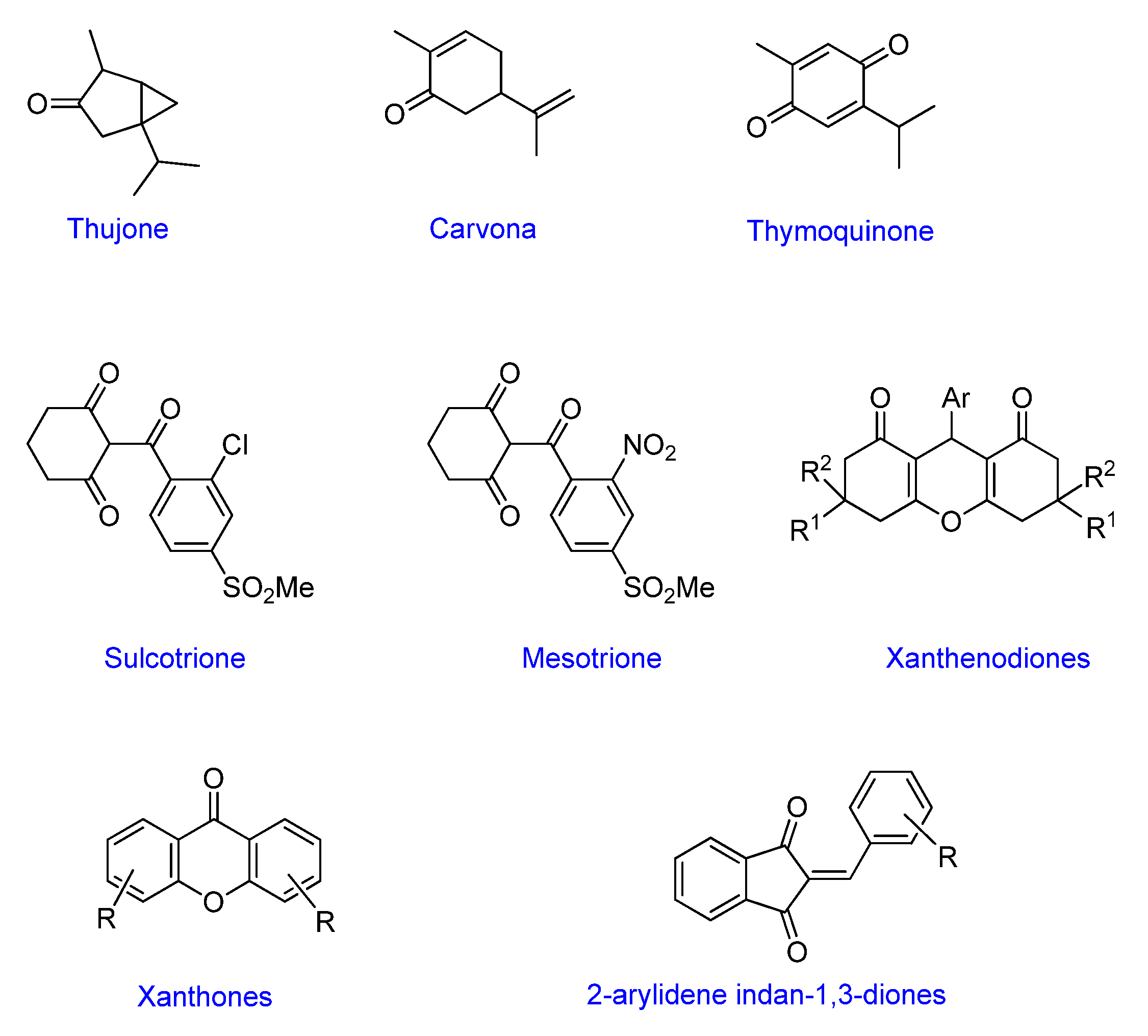
:1. Introduction
2. Materials and Methods
2.1. General Procedure for Preparation of Cyclic Ketones 1–24
2.2. Cell Culture and Viral Stock
2.3. Antiviral Screening Assay
2.4. Viral Inhibition Assay
2.5. Mechanisms of Action Assays
2.5.1. Pre-Treatment Assay
2.5.2. Post-Treatment Assay
2.5.3. Viral Adsorption Inhibition Assay
2.6. Statistical Analysis
3. Results
3.1. Antiviral Screening
3.2. Viral Inhibition Assay
3.3. Mechanism of Action Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Figueiredo, M.L.; Morales, L.T. Review Article Emerging alphaviruses in the Americas: Chikungunya and Mayaro. Rev. Soc. Bras. Med. Trop. 2014, 47, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.R.; Downs, W.G.; Wattley, G.H.; Ahin, N.W.; Reese, A.A. Mayaro virus: A new human disease agent. II. Isolation from blood of patients in Trinidad, B.W.I. Am. J. Trop. Med. Hyg. 1957, 6, 1012–1016. [Google Scholar] [CrossRef]
- Causey, O.R.; Maroja, O.M. Mayaro virus: A new human disease agent. III. Investigation of an epidemic of acute febrile illness on the river Guama in Pará, Brazil, and isolation of Mayaro virus as causative agent. Am. J. Trop. Med. Hyg. 1957, 6, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Mota, M.T.; Ribeiro, M.R.; Vedovello, D.; Nogueira, M.L. Mayaro virus: A neglected arbovirus of the Americas. Future Virol. 2015, 10, 1109–1122. [Google Scholar] [CrossRef]
- Martins, K.A.; Gregory, M.K.; Valdez, S.M.; Sprague, T.R.; Encinales, L.; Pacheco, N.; Cure, C.; Porras-Ramirez, A.; Rico-Mendoza, A.; Chang, A.; et al. Neutralizing antibodies from convalescent chikungunya virus patients can cross-neutralize mayaro and una viruses. Am. J. Trop. Med. Hyg. 2019, 100, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.M.; Dias, R.S.; de Souza Fernandes, L.; da Silva, C.C.; de Paula, S.O. Mayaro Virus Infection: Clinical Features and Global Threat. Curr. Treat. Options Infect. Dis. 2020, 12, 387–397. [Google Scholar] [CrossRef]
- da Silva Pessoa Vieira, C.J.; da Silva, D.J.F.; Barreto, E.S.; Siqueira, C.E.H.; Colombo, T.E.; Ozanic, K.; Schmidt, D.J.; Drumond, B.P.; Mondini, A.; Nogueira, M.L.; et al. Detection of Mayaro virus infections during a dengue outbreak in Mato Grosso, Brazil. Acta Trop. 2015, 147, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Long, K.C.; Ziegler, S.A.; Thangamani, S.; Hausser, N.L.; Kochel, T.J.; Higgs, S.; Tesh, R.B. Experimental transmission of Mayaro virus by Aedes aegypti. Am. J. Trop. Med. Hyg. 2011, 85, 750–757. [Google Scholar] [CrossRef] [Green Version]
- Pereira Serra, O.; Fernandes Cardoso, B.; Maria Ribeiro, A.L.; dos Santos, F.A.L.; Dezengrini Slhessarenko, R. Mayaro virus and dengue virus 1 and 4 natural infection in culicids from Cuiabá, state of Mato Grosso, Brazil. Mem. Inst. Oswaldo Cruz 2016, 111, 20–29. [Google Scholar] [CrossRef]
- Brunini, S.; França, D.D.S.; Silva, J.B.; Silva, L.N.; Silva, F.P.A.; Spadoni, M.; Rezza, G. High frequency of mayaro virus IgM among febrile patients, central Brazil. Emerg. Infect. Dis. 2017, 23, 1025–1026. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.C.; Francy, D.B. Laboratory studies of a Brazilian strain of Aedes albopictus as a potential vector of Mayaro and Oropouche viruses. J. Am. Mosq. Control Assoc. 1991, 7, 89–93. [Google Scholar]
- Lima, W.G.; Pereira, R.S.; da Cruz Nizer, W.S.; Brito, J.C.M.; Godói, I.P.; Cardoso, V.N.; Fernandes, S.O.A.; Ferreira, J.M.S. Rate of exposure to Mayaro virus (MAYV) in Brazil between 1955 and 2018: A systematic review and meta-analysis. Arch. Virol. 2021, 166, 347–361. [Google Scholar] [CrossRef]
- Lorenz, C.; Freitas Ribeiro, A.; Chiaravalloti-Neto, F. Mayaro virus distribution in South America. Acta Trop. 2019, 198, 105093. [Google Scholar] [CrossRef]
- de Souza Costa, M.C.; Siqueira Maia, L.M.; Costa de Souza, V.; Gonzaga, A.M.; Correa de Azevedo, V.; Ramos Martins, L.; Chavez Pavoni, J.H.; Gomes Naveca, F.; Dezengrini Slhessarenko, R. Arbovirus investigation in patients from Mato Grosso during Zika and Chikungunya virus introdution in Brazil, 2015–2016. Acta Trop. 2019, 190, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.L.A.; Fonseca, B.A.L. da Will Mayaro virus be responsible for the next outbreak of an arthropod-borne virus in Brazil? Brazilian J. Infect. Dis. 2017, 21, 540–544. [Google Scholar] [CrossRef]
- Santiago, F.W.; Halsey, E.S.; Siles, C.; Vilcarromero, S.; Guevara, C.; Silvas, J.A.; Ramal, C.; Ampuero, J.S.; Aguilar, P.V. Long-Term Arthralgia after Mayaro Virus Infection Correlates with Sustained Pro-inflammatory Cytokine Response. PLoS Negl. Trop. Dis. 2015, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suchowiecki, K.; Reid, S.P.; Simon, G.L.; Firestein, G.S.; Chang, A. Persistent Joint Pain Following Arthropod Virus Infections. Curr. Rheumatol. Rep. 2021, 23, 987. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.M.; Haese, N.N.; Denton, M.; Ando, T.; Kreklywich, C.; Bonin, K.; Streblow, C.E.; Kreklywich, N.; Smith, P.; Broeckel, R.; et al. Non-replicating adenovirus based mayaro virus vaccine elicits protective immune responses and cross protects against other alphaviruses. PLoS Negl. Trop. Dis. 2021, 15, 1–27. [Google Scholar] [CrossRef]
- Choi, H.; Kudchodkar, S.B.; Reuschel, E.L.; Asijaid, K.; Borole, P.; Ho, M.; Wojtak, K.; Reed, C.; Ramos, S.; Bopp, N.E.; et al. Protective immunity by an engineered DNA vaccine for mayaro virus. PLoS Negl. Trop. Dis. 2019, 13, e0007042. [Google Scholar] [CrossRef]
- Weise, W.J.; Hermance, M.E.; Forrester, N.; Adams, A.P.; Langsjoen, R.; Gorchakov, R.; Wang, E.; Alcorn, M.D.H.; Tsetsarkin, K.; Weaver, S.C. A Novel Live-Attenuated Vaccine Candidate for Mayaro Fever. PLoS Negl. Trop. Dis. 2014, 8, e2969. [Google Scholar] [CrossRef]
- Campos, D.; Navarro, S.; Llamas-González, Y.Y.; Sugasti, M.; González-Santamaría, J. Broad Antiviral Activity of Ginkgolic Acid against Chikungunya, Mayaro, Una, and Zika Viruses. Viruses 2020, 12, 449. [Google Scholar] [CrossRef] [Green Version]
- Amorim, R.; de Meneses, M.D.F.; Borges, J.C.; da Silva Pinheiro, L.C.; Caldas, L.A.; Cirne-Santos, C.C.; de Mello, M.V.P.; de Souza, A.M.T.; Castro, H.C.; de Palmer Paixão, I.C.N.; et al. Thieno[2,3-b]pyridine derivatives: A new class of antiviral drugs against Mayaro virus. Arch. Virol. 2017, 162, 1577–1587. [Google Scholar] [CrossRef]
- Ferreira, P.G.; Ferraz, A.C.; Figueiredo, J.E.; Lima, C.F.; Rodrigues, V.G.; Taranto, A.G.; Ferreira, J.M.S.; Brandão, G.C.; Vieira-Filho, S.A.; Duarte, L.P.; et al. Detection of the antiviral activity of epicatechin isolated from Salacia crassifolia (Celastraceae) against Mayaro virus based on protein C homology modelling and virtual screening. Arch. Virol. 2018, 163, 1567–1576. [Google Scholar] [CrossRef]
- Camini, F.C.; da Silva, T.F.; da Silva Caetano, C.C.; Almeida, L.T.; Ferraz, A.C.; Alves Vitoreti, V.M.; de Mello Silva, B.; de Queiroz Silva, S.; de Magalhães, J.C.; de Brito Magalhães, C.L. Antiviral activity of silymarin against Mayaro virus and protective effect in virus-induced oxidative stress. Antiviral Res. 2018, 158, 8–12. [Google Scholar] [CrossRef]
- Ferraz, A.C.; Moraes, T.D.; da Cruz Nizer, W.S.; Dos Santos, M.; Totola, A.H.; Ferreira, J.M.; Vieira-Filho, S.A.; Rodrigues, V.G.; Duarte, L.P.; de Brito Magalhaes, C.L.; et al. Virucidal activity of proanthocyanidin against Mayaro virus. Antiviral Res. 2019, 168, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Spindola, K.C.W.; Simas, N.K.; Salles, T.S.; De Meneses, M.D.F.; Sato, A.; Ferreira, D.; Romão, W.; Kuster, R.M. Anti-Mayaro virus activity of Cassia australis extracts (Fabaceae, Leguminosae). Parasites Vectors 2014, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sivropoulou, A.; Nikolaou, C.; Papanikolaou, E.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antimicrobial, Cytotoxic, and Antiviral Activities of Salvia fructicosa Essential Oil. J. Agric. Food Chem. 1997, 45, 3197–3201. [Google Scholar] [CrossRef]
- Chung, M.S. Antiviral activities of Artemisia princeps var. orientalis essential oil and its α-thujone against norovirus surrogates. Food Sci. Biotechnol. 2017, 26, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Pombal, S.; Hernández, Y.; Diez, D.; Mondolis, E.; Mero, A.; Morán-Pinzón, J.; Guerrero, E.I.; Rodilla, J.M. Antioxidant activity of carvone and derivatives against superoxide ion. Nat. Prod. Commun. 2017, 12, 653–655. [Google Scholar] [CrossRef] [Green Version]
- Pizzolitto, R.P.; Herrera, J.M.; Zaio, Y.P.; Dambolena, J.S.; Zunino, M.P.; Gallucci, M.N.; Zygadlo, J.A. Bioactivities of ketones terpenes: Antifungal effect on f. verticillioides and repellents to control insect fungal vector, s. zeamais. Microorganisms 2015, 3, 851–865. [Google Scholar] [CrossRef]
- Jeschke, P.; Witschel, M.; Krämer, W.; Schirmer, U. Herbicides with Bleaching Properties. In Modern Crop Protection Compounds; John Wiley & Sons, Ltd.: Weinheim, Germany, 2019; Volume 3, pp. 213–302. ISBN 9783527699261. [Google Scholar]
- de Souza, A.P.M.; Costa, M.C.A.; de Aguiar, A.R.; Bressan, G.C.; de Almeida Lima, G.D.; Lima, W.P.; Borsodi, M.P.G.; Bergmann, B.R.; Ferreira, M.M.C.; Teixeira, R.R. Leishmanicidal and cytotoxic activities and 4D-QSAR of 2-arylidene indan-1,3-diones. Arch. Pharm. 2021, 354, e2100081. [Google Scholar] [CrossRef]
- da Silva, M.L.; Teixeira, R.R.; de Azevedo Santos, L.; Martins, F.T.; Ramalho, T.C. Synthesis and special characterization through X-ray analysis of 1,8-dioxooctahydroxanthenes. Arab. J. Chem. 2017, 13, 974–987. [Google Scholar] [CrossRef]
- de Jesus Menezes, A.P.; da Silva, M.L.; Pereira, W.L.; de Paula Costa, G.; Horta, A.L.; Mendonça, A.A.S.; Carneiro, A.C.A.; de Souza, D.M.S.; Novaes, R.D.; Teixeira, R.R.; et al. In vitro tripanocidal effect of 1,8-dioxooctahydroxanthenes (xanthenodiones) and tetraketones and improvement of cardiac parameters in vivo. J. Glob. Antimicrob. Resist. 2020, 22, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Shagufta, J.; Ahmad, I. Recent insight into the biological activities of synthetic xanthone derivatives. Eur. J. Med. Chem. 2016, 116, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.F.C.d.S.; de Souza, A.P.M.; de Oliveira, A.S.; da Silva, M.L.; de Oliveira, F.M.; Santos, E.G.; da Silva, Í.E.P.; Ferreira, R.S.; Villela, F.S.; Martins, F.T.; et al. Zirconium catalyzed synthesis of 2-arylidene Indan-1,3-diones and evaluation of their inhibitory activity against NS2B-NS3 WNV protease. Eur. J. Med. Chem. 2018, 149, 98–109. [Google Scholar] [CrossRef]
- da Silva, Í.E.P.; Lopes da Silva, M.; Dias, R.S.; Santos, E.G.; Brangioni de Paula, M.C.; Silva de Oliveira, A.; Costa da Silveira Oliveira, A.F.; Marques de Oliveira, F.; Canedo da Silva, C.; Teixeira, R.R.; et al. Xanthenedione (and intermediates involved in their synthesis) inhibit Zika virus migration to the central nervous system in murine neonatal models. Microbes Infect. 2020, 22, 489–499. [Google Scholar] [CrossRef]



| Compound | Structure | Compound | Structure |
|---|---|---|---|
| 1 |  | 7 |  |
| 2 |  | 8 |  |
| 3 |  | 9 |  |
| 4 |  | 10 |  |
| 5 |  | 11 |  |
| 6 |  | 12 |  |
| 13 |  | 20 |  |
| 14 |  | 21 |  |
| 15 |  | 22 |  |
| 16 |  | 23 |  |
| 17 |  | 24 |  |
| 18 |  |
| Compound | CC50 (µmol·L−1) | EC50 (µmol·L−1) | SI |
|---|---|---|---|
| 3 | 292.6 ± 16.3 | 45.7 ± 13.4 | 6.4 |
| 5 | 27.6 ± 7.1 | 87.1 ± 32.3 | 0.3 |
| 7 | 91.2 ± 9.4 | 8.4 ± 2.0 | 10.8 |
| 8 | 336.8 ± 50.5 | 65.3 ± 15.8 | 5.2 |
| 9 | 338.8 ± 53.9 | 21.5 ± 6.5 | 15.8 |
| 10 | 324.1 ± 100.6 | nd 1 | nd |
| 12 | 345.4 ± 47.4 | 77.1 ± 40.2 | 4.8 |
| 15 | 343.7 ± 86.3 | nd | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, L.S.; da Silva, M.L.; Dias, R.S.; da S. Lucindo, M.S.; da Silva, Í.E.P.; Silva, C.C.; Teixeira, R.R.; de Paula, S.O. Evaluation of Antiviral Activity of Cyclic Ketones against Mayaro Virus. Viruses 2021, 13, 2123. https://doi.org/10.3390/v13112123
Fernandes LS, da Silva ML, Dias RS, da S. Lucindo MS, da Silva ÍEP, Silva CC, Teixeira RR, de Paula SO. Evaluation of Antiviral Activity of Cyclic Ketones against Mayaro Virus. Viruses. 2021; 13(11):2123. https://doi.org/10.3390/v13112123
Chicago/Turabian StyleFernandes, Luciana S., Milene L. da Silva, Roberto S. Dias, Marcel S. da S. Lucindo, Ítalo E. P. da Silva, Cynthia C. Silva, Róbson R. Teixeira, and Sérgio O. de Paula. 2021. "Evaluation of Antiviral Activity of Cyclic Ketones against Mayaro Virus" Viruses 13, no. 11: 2123. https://doi.org/10.3390/v13112123






