Testing a Recombinant Form of Tetanus Toxoid as a Carrier Protein for Glycoconjugate Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Polysaccharides
2.2. Proteins
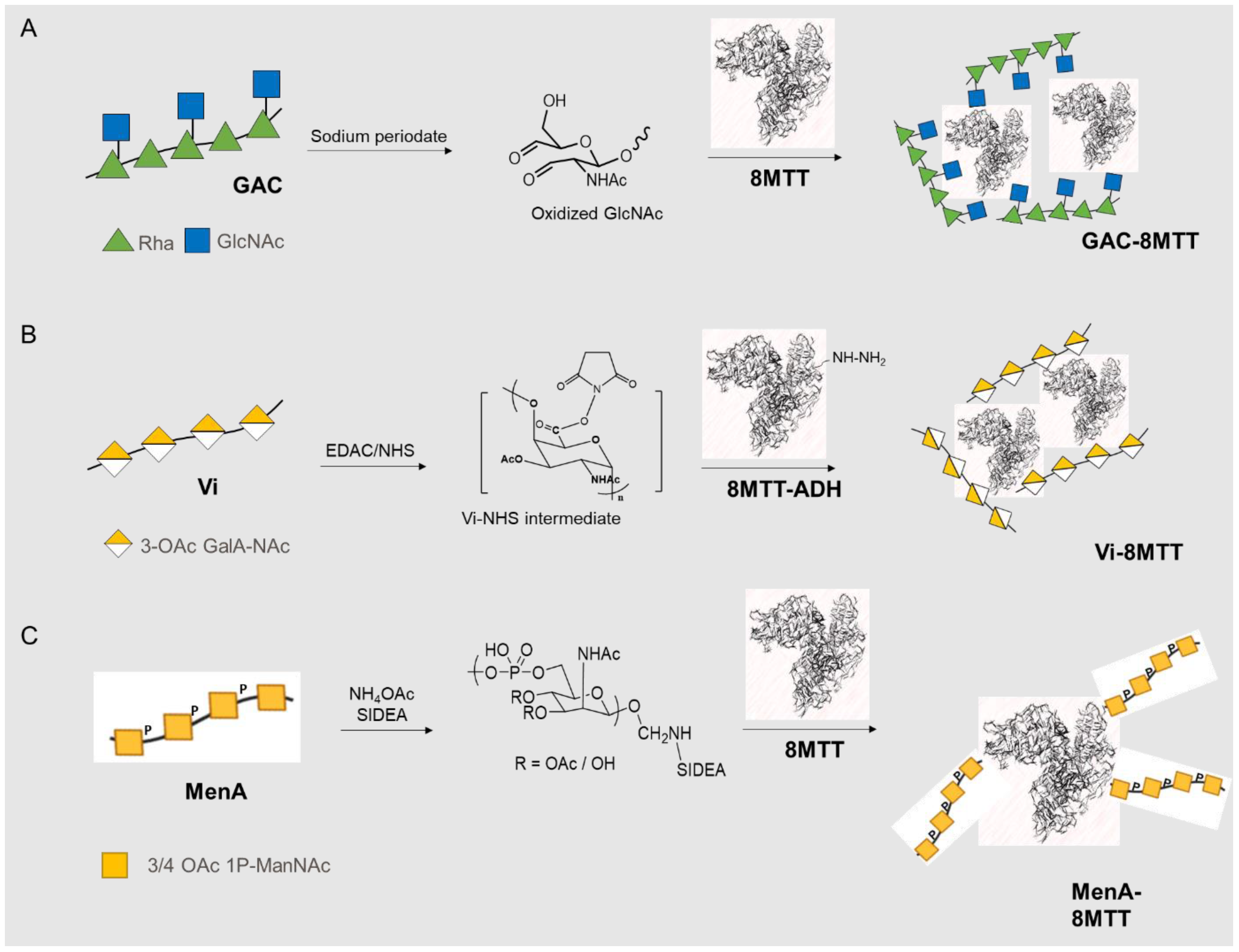
2.3. Glycoconjgates Synthesis
2.3.1. GAC Glycoconjugates
2.3.2. Vi Glycoconjugates
2.3.3. Men Glycoconjugates
2.4. Glycoconjugates Characterization
2.5. Immunogenicity Studies in Mice and Assessment of Antibody Responses
2.6. Statistics
3. Results
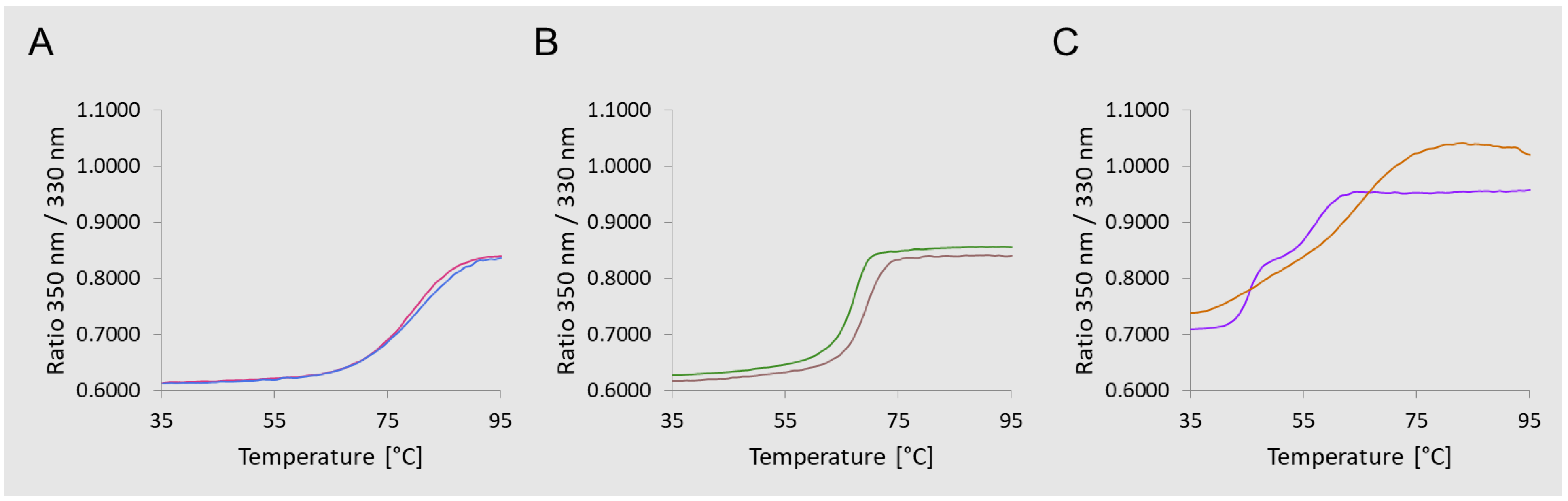
3.1. Glycoconjugates Preparation
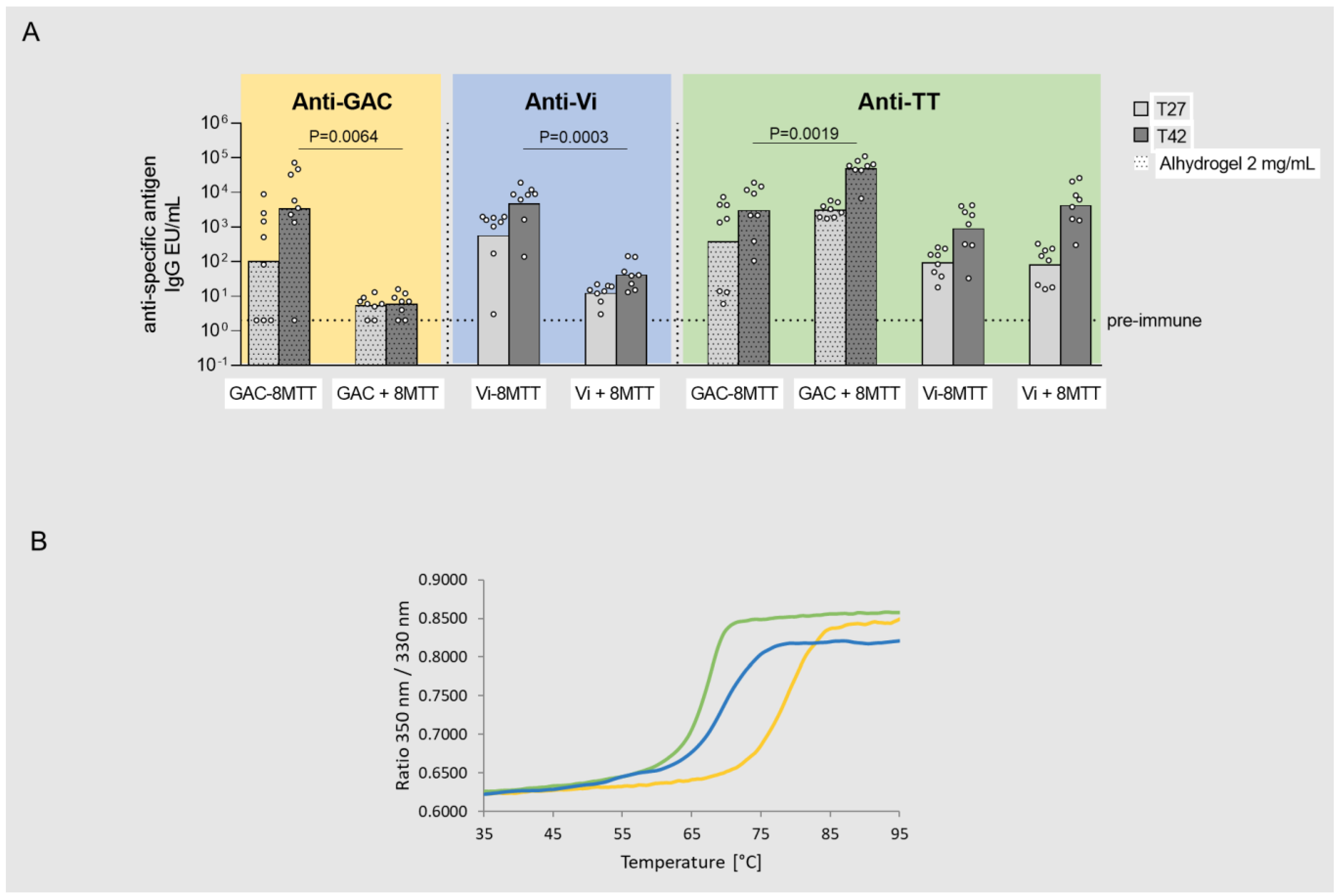
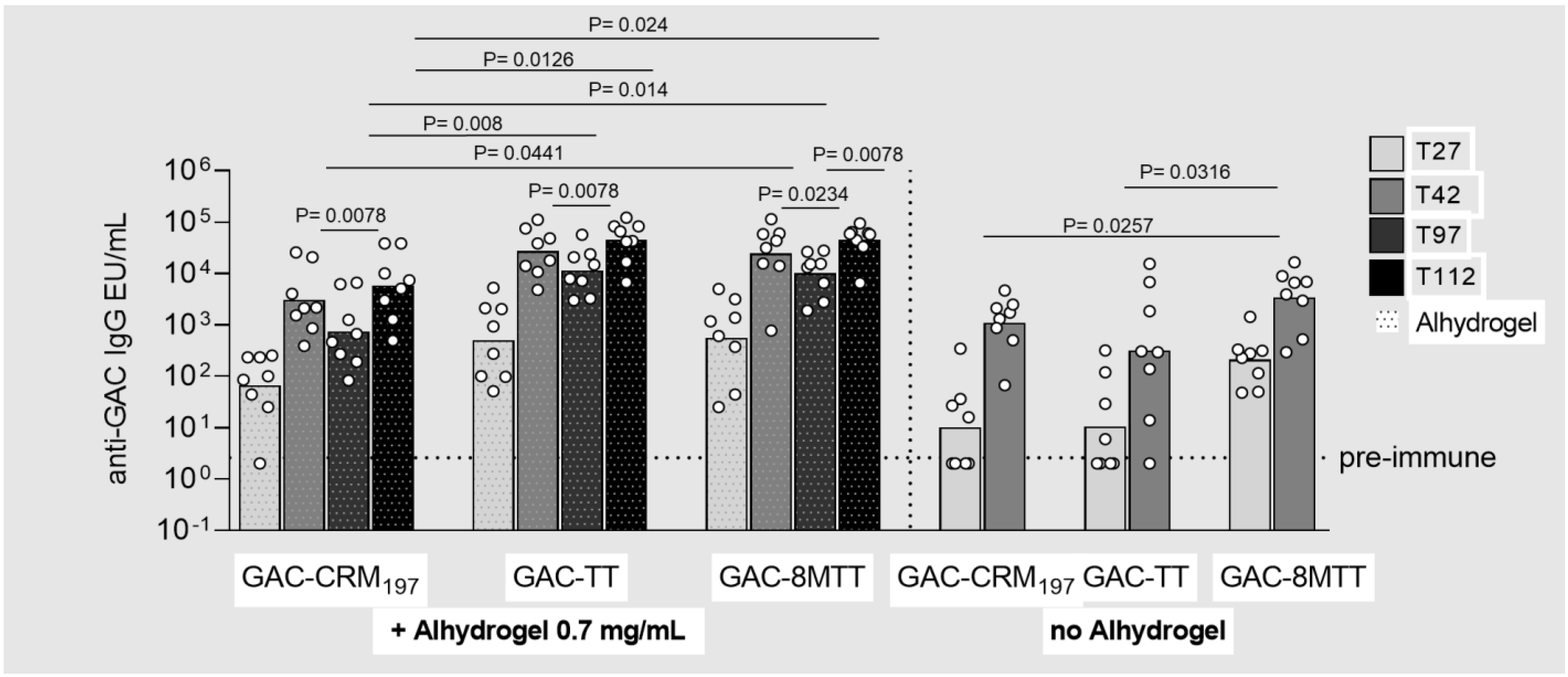
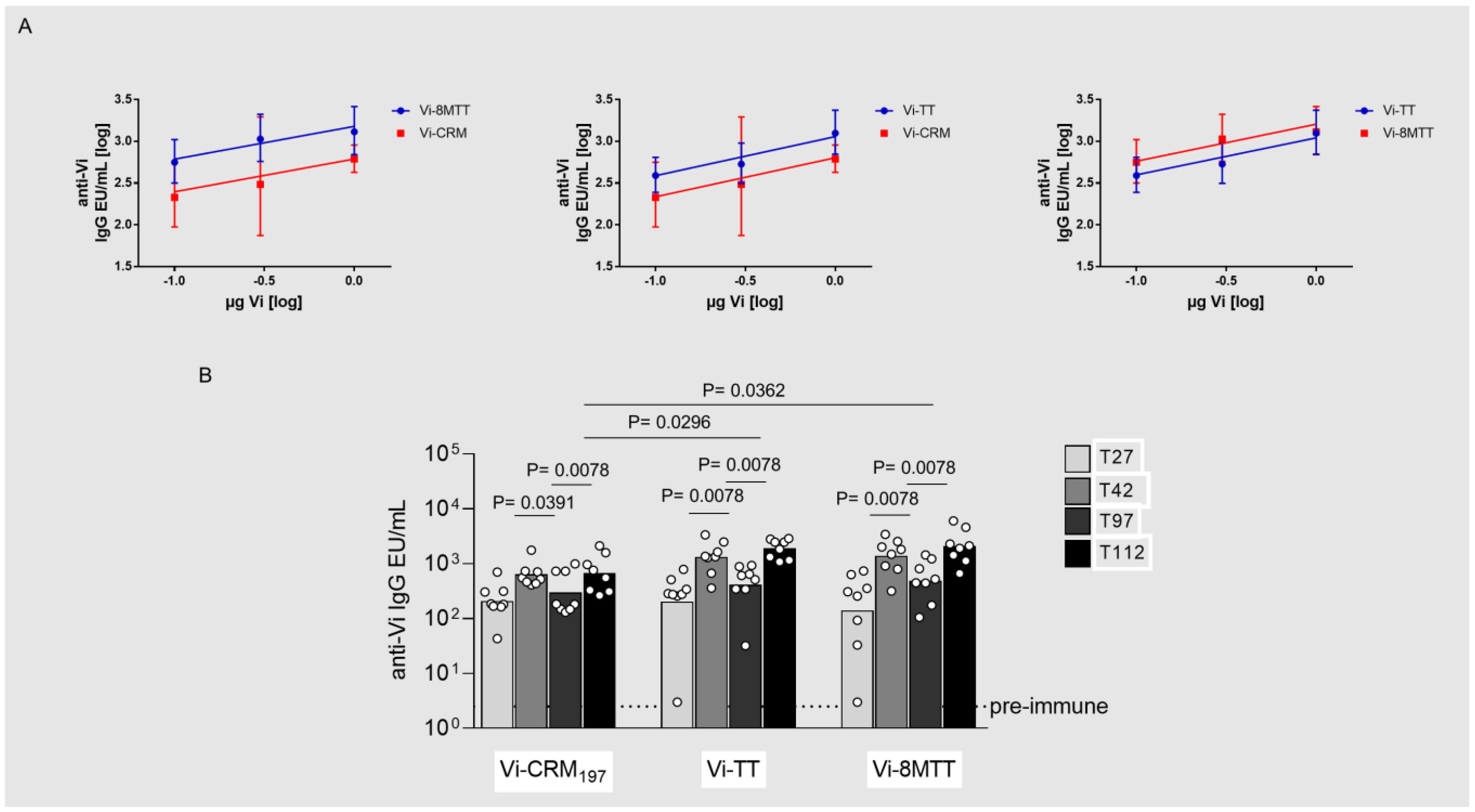
3.2. Immunogenicity Studies in Mice with GAC and Vi Glycoconjugates, Comparing 8MTT to CRM197 and TT Carrier Proteins
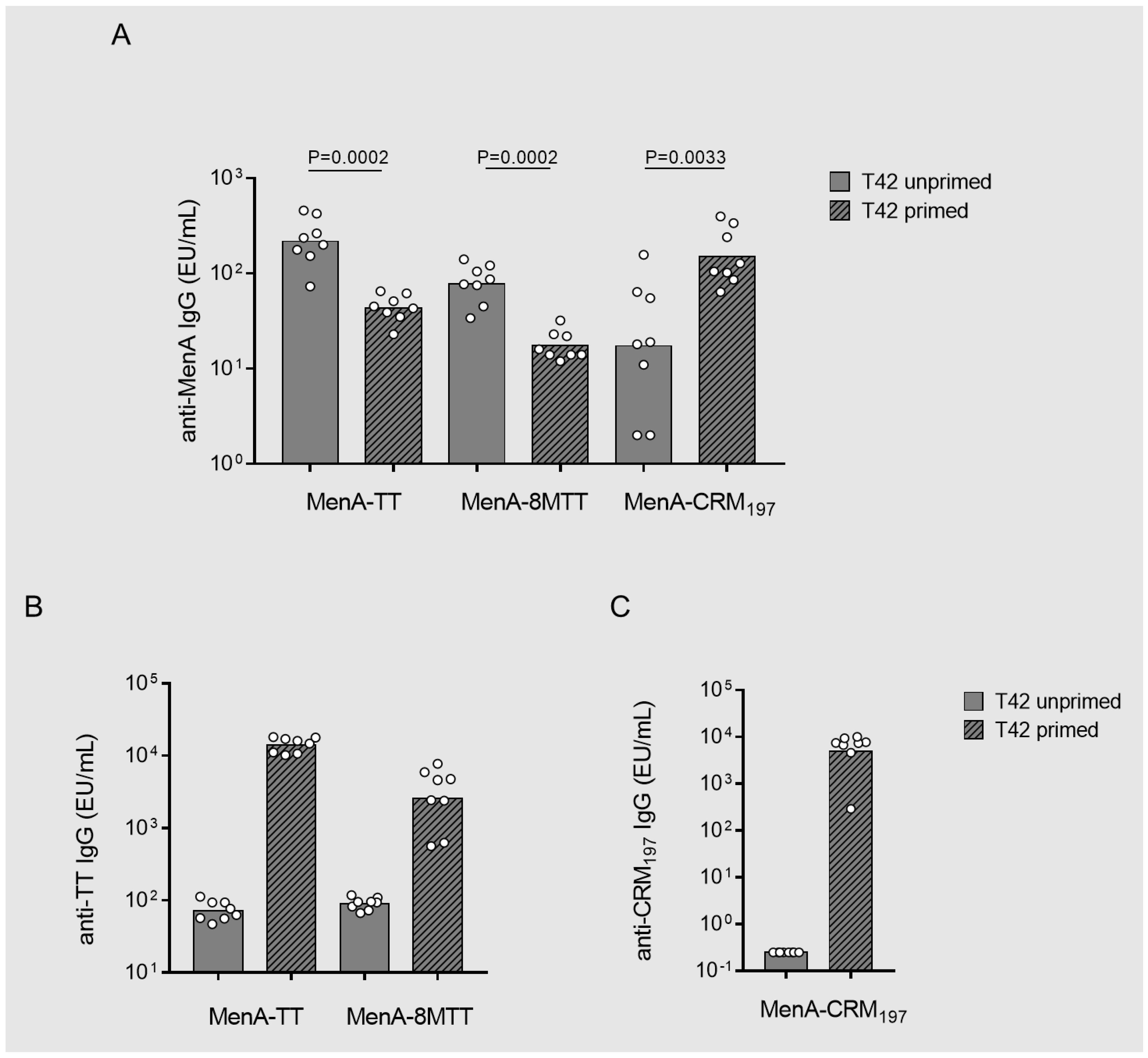
3.3. Carrier-Priming Effect on the Immune Response Elicited in Mice by MenA Glycoconjugates
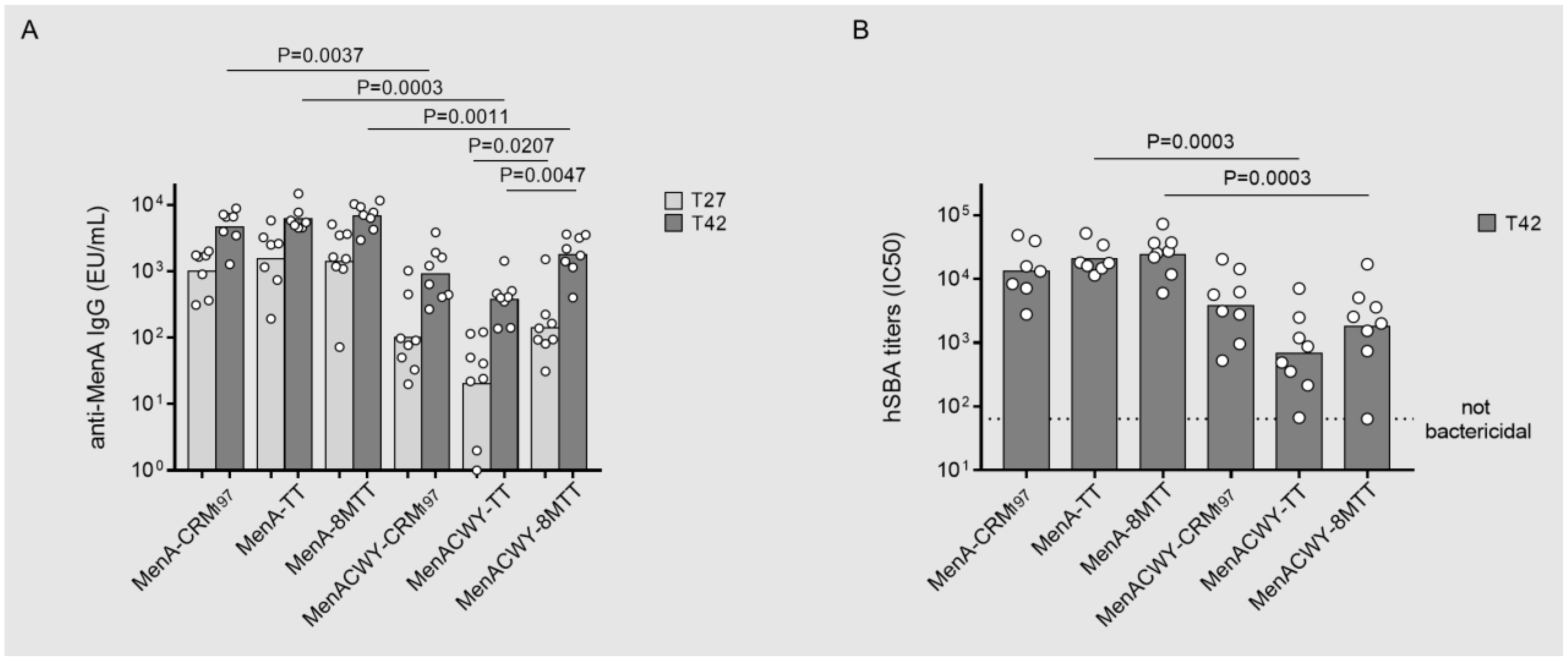
3.4. Immunointerference on Anti-MenA Response in MenACWY Tetravalent Formulations by Using 8MTT, TT or CRM197 as Carrier Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avery, O.T.; Goebel, W.F. Chemo-Immunological Studie on Conjugated Carbohydrate-Proteins. J. Exp. Med. 1931, 54, 437–447. [Google Scholar] [CrossRef]
- Adamo, R. Glycoconjugate Vaccines: Classic and Novel Approaches. Glycoconj. J. 2021, 38, 397–398. [Google Scholar] [CrossRef]
- van der Put, R.M.F.; Metz, B.; Pieters, R.J. Carriers and Antigens: New Developments in Glycoconjugate Vaccines. Vaccines 2023, 11, 219. [Google Scholar] [CrossRef]
- Sorieul, C.; Dolce, M.; Romano, M.R.; Codee, J.; Adamo, R. Glycoconjugate Vaccines against Antimicrobial Resistant Pathogens. Expert Rev. Vaccines 2023, 22, 1055–1078. [Google Scholar] [CrossRef]
- Vella, M.; Pace, D. Glycoconjugate Vaccines: An Update. Expert Opin. Biol. Ther. 2015, 15, 529–546. [Google Scholar] [CrossRef]
- Schneerson, R.; Barbera, O.; Sutton, A.; Robbins, J.B. Preparation, Characterization and Immunogenicity of Haemophilus Influenzae Type B Polysaccharide Protein Conjugates. J. Exp. Med. 1980, 152, 361–376. [Google Scholar] [CrossRef]
- Duke, J.A.; Avci, F.Y. Immunological Mechanisms of Glycoconjugate Vaccines. In Carbohydrate-Based Vaccines: From Concept to Clinic; American Chemical Society: Washington, DC, USA, 2018; pp. 61–74. [Google Scholar]
- Mond, J.J.; Vos, Q.; Lees, A.; Snapper, C.M. T Cell Indipendent Antigens. Curr. Opin. Immunol. 1995, 7, 349–354. [Google Scholar] [CrossRef]
- Anish, C.; Beurret, M.; Poolman, J. Combined Effects of Glycan Chain Length and Linkage Type on the Immunogenicity of Glycoconjugate Vaccines. NPJ Vaccines 2021, 6, 150. [Google Scholar] [CrossRef]
- Khatun, F.; Stephenson, R.J.; Toth, I. An Overview of Structural Features of Antibacterial Glycoconjugate Vaccines That Influence Their Immunogenicity. Chemistry 2017, 23, 4233–4254. [Google Scholar] [CrossRef]
- Pichichero, M.E. Protein Carriers of Conjugate Vaccines: Characteristics, Development, and Clinical Trials. Hum. Vaccines Immunother. 2013, 9, 2505–2523. [Google Scholar] [CrossRef] [PubMed]
- Neumuller, C. Detoxification of Diphteria Toxin with Formaldehyde Mixed with an Amino-Acid. Nature 1954, 174, 405–406. [Google Scholar] [CrossRef]
- Khatuntseva, E.A.; Nifantiev, N.E. Cross Reacting Material (Crm197) as a Carrier Protein for Carbohydrate Conjugate Vaccines Targeted at Bacterial and Fungal Pathogens. Int. J. Biol. Macromol. 2022, 218, 775–798. [Google Scholar] [CrossRef]
- Forsgren, A.; Riesbeck, K.; Janson, H. Protein D of Haemophilus Influenzae: A Protective Nontypeable H. Influenzae Antigen and a Carrier for Pneumococcal Conjugate Vaccines. Clin. Infect. Dis. 2008, 46, 726–731. [Google Scholar] [CrossRef]
- Donnelly, J.J.; Deck, R.R.; Liu, M.A. Immunogenicity of a Haemophilus Influenzae Polysaccharide-Neisseria Meningitidis Outer Membrane Protein Complex Conjugate Vaccine. J. Immunol. 1990, 145, 3071–3079. [Google Scholar] [CrossRef]
- Kilpi, T.; Ahman, H.; Jokinen, J. Protective Efficacy of a Second Pneumococcal Conjugate Vaccine against Pneumococcal Acute Otitis Media in Infants and Children: Randomized, Controlled Trial of a 7-Valent Pneumococcal Polysaccharide–Meningococcal Outer Membrane Protein Complex Conjugate Vaccine in 1666 Children. Clin. Infect. Dis. 2003, 37, 1155–1164. [Google Scholar]
- Gupta, S.; Pellett, S. Recent Developments in Vaccine Design: From Live Vaccines to Recombinant Toxin Vaccines. Toxins 2023, 15, 563. [Google Scholar] [CrossRef]
- Behrensdorf-Nicol, H.A.; Kegel, B.; Bonifas, U.; Silberbach, K.; Klimek, J.; Weiber, K.; Kramer, B. Residual Enzymatic Activity of the Tetanus Toxin Light Chain Present in Tetanus Toxoid Batches Used for Vaccine Production. Vaccine 2008, 26, 3835–3841. [Google Scholar] [CrossRef]
- Stojicevic, I.; Dimitrijevic, L.; Dovezenski, N.; Zivkovic, I.; Petrusic, V.; Marinkovic, E.; Inic-Kanada, A.; Stojanovic, M. Tetanus Toxoid Purification: Chromatographic Procedures as an Alternative to Ammonium-Sulphate Precipitation. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Broker, M.; Costantino, P.; DeTora, L.; McIntosh, E.D.; Rappuoli, R. Biochemical and Biological Characteristics of Cross-Reacting Material 197 Crm197, a Non-Toxic Mutant of Diphtheria Toxin: Use as a Conjugation Protein in Vaccines and Other Potential Clinical Applications. Biologicals 2011, 39, 195–204. [Google Scholar] [CrossRef]
- Hickey, J.M.; Toprani, V.M.; Kaur, K.; Mishra, R.P.N.; Goel, A.; Oganesyan, N.; Lees, A.; Sitrin, R.; Joshi, S.B.; Volkin, D.B. Analytical Comparability Assessments of 5 Recombinant Crm(197) Proteins from Different Manufacturers and Expression Systems. J. Pharm. Sci. 2018, 107, 1806–1819. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.T.; Oganesyan, N.; Lees, A. Monomeric Crystal Structure of the Vaccine Carrier Protein Crm(197) and Implications for Vaccine Development. Acta Crystallogr. F Struct. Biol. Commun. 2023, 79, 82–86. [Google Scholar] [CrossRef]
- Przedpelski, A.; Tepp, W.H.; Pellett, S.; Johnson, E.A.; Barbieri, J.T. A Novel High-Potency Tetanus Vaccine. mBio 2020, 11, 10–128. [Google Scholar] [CrossRef]
- Chang, M.J.; Ollivault-Shiflett, M.; Schuman, R.; Nguyen, S.N.; Kaltashov, I.A.; Bobst, C.; Rajagopal, S.P.; Przedpelski, A.; Barbieri, J.T.; Lees, A. Genetically Detoxified Tetanus Toxin as a Vaccine and Conjugate Carrier Protein. Vaccine 2022, 40, 5103–5113. [Google Scholar] [CrossRef]
- Wang, J.; Ma, C.; Li, M.; Gao, X.; Wu, H.; Dong, W.; Wei, L. Streptococcus Pyogenes: Pathogenesis and the Current Status of Vaccines. Vaccines 2023, 11, 1510. [Google Scholar] [CrossRef]
- Szu, S.; Taylor, D.N.; Trofa, A.C.; Clements, J.D.; Shiloach, J.; Sadof, J.C.; Bryla, D.A.; Robbins, J.B. Laboratory and Preliminary Clinical Characterization of Vi Capsular Polysaccharide-Protein Conjugate Vaccines. Infect. Immun. 1994, 62, 4440–4444. [Google Scholar] [CrossRef]
- Szu, S.C. Development of Vi Conjugate—A New Generation of Typhoid Vaccine. Expert Rev. Vaccines 2013, 12, 1273–1286. [Google Scholar] [CrossRef]
- Gasparini, R.; Panatto, D. Meningococcal Glycoconjugate Vaccines. Hum. Vaccines 2011, 7, 170–182. [Google Scholar] [CrossRef]
- Pace, D.; Pollard, A.J.; Messonier, N.E. Quadrivalent Meningococcal Conjugate Vaccines. Vaccine 2009, 27 (Suppl. 2), B30–B41. [Google Scholar] [CrossRef] [PubMed]
- Pobre, K.; Tashani, M.; Ridda, I.; Rashid, H.; Wong, M.; Booy, R. Carrier Priming or Suppression: Understanding Carrier Priming Enhancement of Anti-Polysaccharide Antibody Response to Conjugate Vaccines. Vaccine 2014, 32, 1423–1430. [Google Scholar] [CrossRef]
- Knuf, M.; Kowalzik, F.; Kieninger, D. Comparative Effects of Carrier Proteins on Vaccine-Induced Immune Response. Vaccine 2011, 29, 4881–4890. [Google Scholar] [CrossRef]
- Borrow, R.; Dagan, R.; Zepp, F.; Hallander, H.; Poolman, J. Glycoconjugate Vaccines and Immune Interactions, and Implications for Vaccination Schedules. Expert Rev. Vaccines 2011, 10, 1621–1631. [Google Scholar] [CrossRef]
- Findlow, H.; Borrow, R. Interactions of Conjugate Vaccines and Co-Administered Vaccines. Hum. Vaccines Immunother. 2016, 12, 226–230. [Google Scholar] [CrossRef]
- Pecetta, S.; Surdo, P.L.; Tontini, M.; Proietti, D.; Zambonelli, C.; Bottomley, M.J.; Biagini, M.; Berti, F.; Costantino, P.; Romano, M.R.; et al. Carrier Priming with Crm 197 or Diphtheria Toxoid Has a Different Impact on the Immunogenicity of the Respective Glycoconjugates: Biophysical and Immunochemical Interpretation. Vaccine 2015, 33, 314–320. [Google Scholar] [CrossRef]
- Palmieri, E.; Kis, Z.; Ozanne, J.; Di Benedetto, R.; Ricchetti, B.; Massai, L.; Carducci, M.; Oldrini, D.; Gasperini, G.; Aruta, M.G.; et al. Gmma as an Alternative Carrier for a Glycoconjugate Vaccine against Group a Streptococcus. Vaccines 2022, 10, 1034. [Google Scholar] [CrossRef]
- Arcuri, M.; Di Benedetto, R.; Cunningham, A.F.; Saul, A.; MacLennan, C.A.; Micoli, F. The Influence of Conjugation Variables on the Design and Immunogenicity of a Glycoconjugate Vaccine against Salmonella Typhi. PLoS ONE 2017, 12, e0189100. [Google Scholar] [CrossRef]
- De Ricco, R.; Rech, F.; Onnis, V.; Coccone, S.S.; Scalia, G.; Marcozzi, C.; Gavini, M.; Beni, S.; Giannini, S.; Nompari, L.; et al. Development of a New Solid-Phase Extraction Base Method for Free Saccharide Content Estimation of Meningococcal Conjugate Vaccines. ACS Omega 2022, 7, 39875–39883. [Google Scholar] [CrossRef]
- Lei, Q.P.; Lamb, D.H.; Heller, R.; Pietrobon, P. Quantitation of Low Level Unconjugated Polysaccharide in Tetanus Toxoid-Conjugate Vaccine by Hpaec/Pad Following Rapid Separation by Deoxycholate/Hcl. J. Pharm. Biomed. Anal. 2000, 21, 1087–1091. [Google Scholar] [CrossRef]
- Borrow, R.; Aaberge, I.S.; Santos, G.F.; Eudey, T.L.; Oster, P.; Glennie, A.; Findlow, J.; Hoiby, E.A.; Rosenqvist, E.; Balmer, P.; et al. Interlaboratory Standardization of the Measurement of Serum Bactericidal Activity by Using Human Complement against Meningococcal Serogroup B, Strain 44/76-Sl, before and after Vaccination with the Norwegian Menbvac Outer Membrane Vesicle Vaccine. Clin. Diagn. Lab. Immunol. 2005, 12, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Rondini, S.; Pisoni, I.; Proietti, D.; Berti, F.; Costantino, P.; Rappuoli, R.; Szu, S.; Saul, A.; Martin, L.B. Vi-Crm 197 as a New Conjugate Vaccine against Salmonella Typhi. Vaccine 2011, 29, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Oerlemans, R.; Groves, M.R. Theory and Applications of Differential Scanning Fluorimetry in Early-Stage Drug Discovery. Biophys. Rev. 2020, 12, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Ramon, G. Method of Anatoxin Vaccination; Vaccination against Diphtheria and Tetanus. Development and Results. Srp. Arh. Celok. Lek. 1954, 82, 1173–1192. [Google Scholar] [PubMed]
- Cui, C.; Carbis, R.; An, S.J.; Jang, H.; Czerkinsky, C.; Szu, S.C.; Clemens, J.D. Physical and Chemical Characterization and Immunologic Properties of Salmonella Enterica Serovar Typhi Capsular Polysaccharide-Diphtheria Toxoid Conjugates. Clin. Vaccine Immunol. 2010, 17, 73–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Avci, F.Y.; Li, X.; Tsuji, M.; Kasper, D.L. A Mechanism for Glycoconjugate Vaccine Activation of the Adaptive Immune System and Its Implications for Vaccine Design. Nat. Med. 2011, 17, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Broker, M. Potential Protective Immunogenicity of Tetanus Toxoid, Diphtheria Toxoid and Cross Reacting Material 197 (Crm197) When Used as Carrier Proteins in Glycoconjugates. Hum. Vaccines Immunother. 2016, 12, 664–667. [Google Scholar] [CrossRef]
- Dagan, R.; Poolman, J.; Siegrist, C.A. Glycoconjugate Vaccines and Immune Interference: A Review. Vaccine 2010, 28, 5513–5523. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oldrini, D.; Di Benedetto, R.; Carducci, M.; De Simone, D.; Massai, L.; Alfini, R.; Galli, B.; Brunelli, B.; Przedpelski, A.; Barbieri, J.T.; et al. Testing a Recombinant Form of Tetanus Toxoid as a Carrier Protein for Glycoconjugate Vaccines. Vaccines 2023, 11, 1770. https://doi.org/10.3390/vaccines11121770
Oldrini D, Di Benedetto R, Carducci M, De Simone D, Massai L, Alfini R, Galli B, Brunelli B, Przedpelski A, Barbieri JT, et al. Testing a Recombinant Form of Tetanus Toxoid as a Carrier Protein for Glycoconjugate Vaccines. Vaccines. 2023; 11(12):1770. https://doi.org/10.3390/vaccines11121770
Chicago/Turabian StyleOldrini, Davide, Roberta Di Benedetto, Martina Carducci, Daniele De Simone, Luisa Massai, Renzo Alfini, Barbara Galli, Brunella Brunelli, Amanda Przedpelski, Joseph T. Barbieri, and et al. 2023. "Testing a Recombinant Form of Tetanus Toxoid as a Carrier Protein for Glycoconjugate Vaccines" Vaccines 11, no. 12: 1770. https://doi.org/10.3390/vaccines11121770
APA StyleOldrini, D., Di Benedetto, R., Carducci, M., De Simone, D., Massai, L., Alfini, R., Galli, B., Brunelli, B., Przedpelski, A., Barbieri, J. T., Rossi, O., Giannelli, C., Rappuoli, R., Berti, F., & Micoli, F. (2023). Testing a Recombinant Form of Tetanus Toxoid as a Carrier Protein for Glycoconjugate Vaccines. Vaccines, 11(12), 1770. https://doi.org/10.3390/vaccines11121770










