Applicability of Information Theory to the Quantification of Responses to Anthropogenic Noise by Southeast Alaskan Humpback Whales
Abstract
:1. Introduction
2. Materials and Methods for Humpback Whale Signal Collection
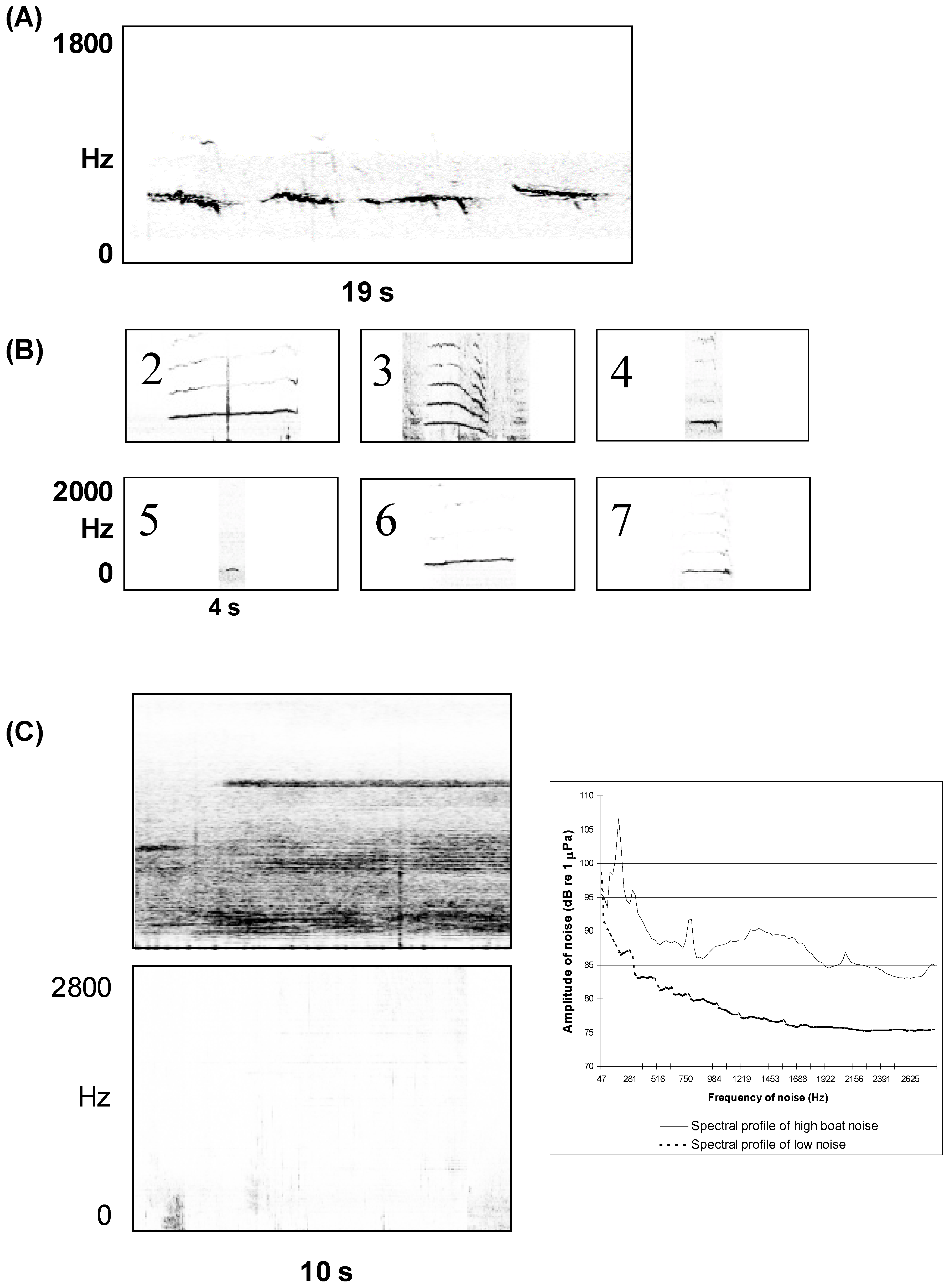
3. Quantifying Humpback Whale Vocal Responses to Boat Noise
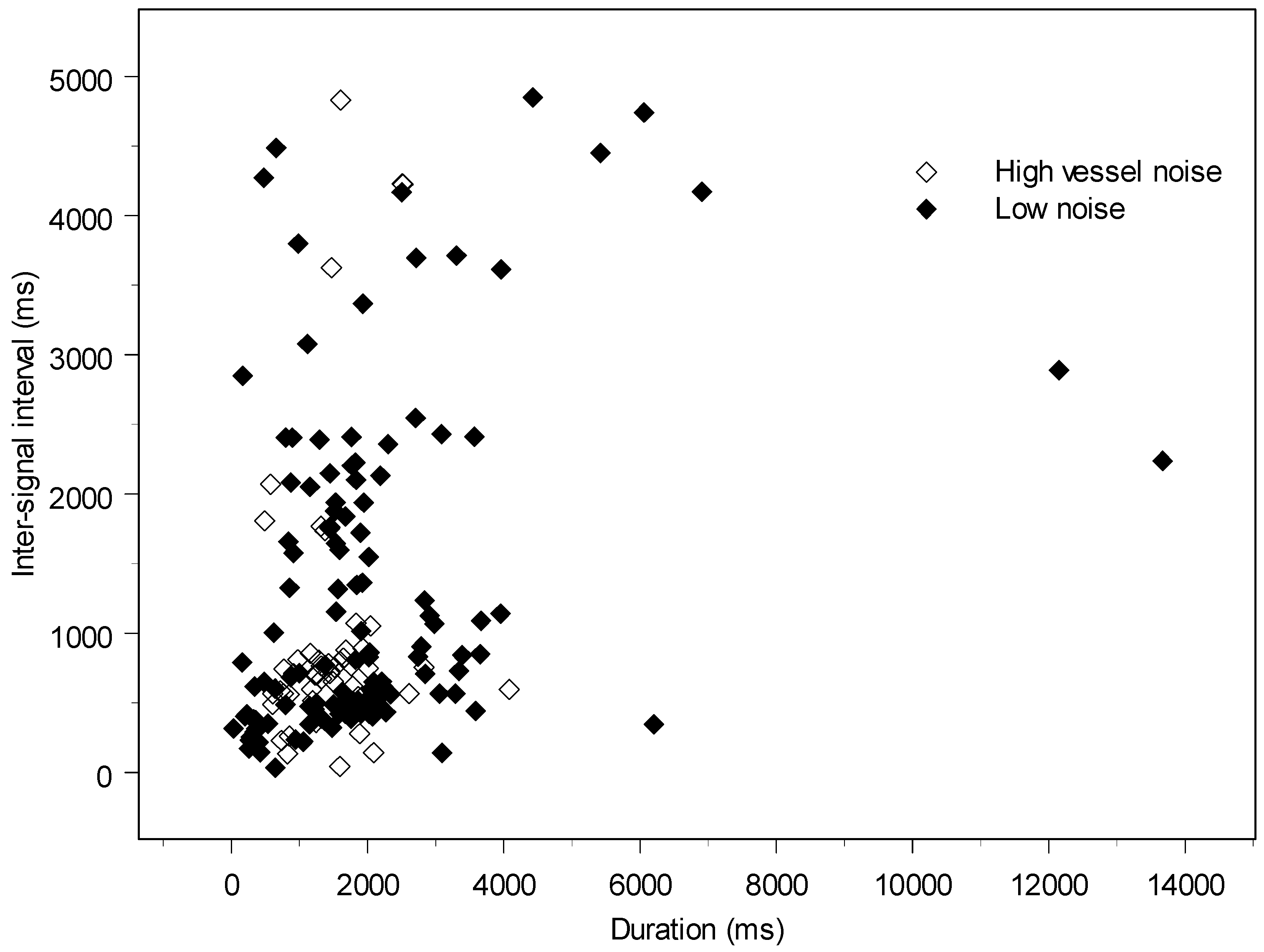
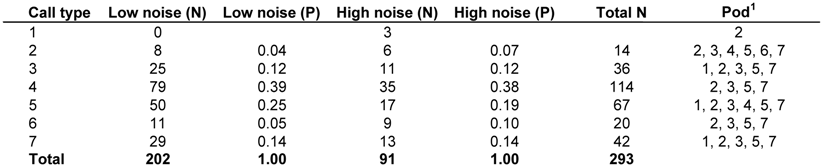 |
 |
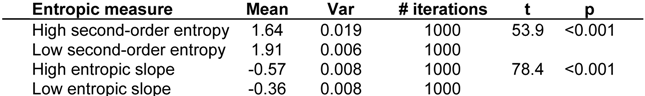 |
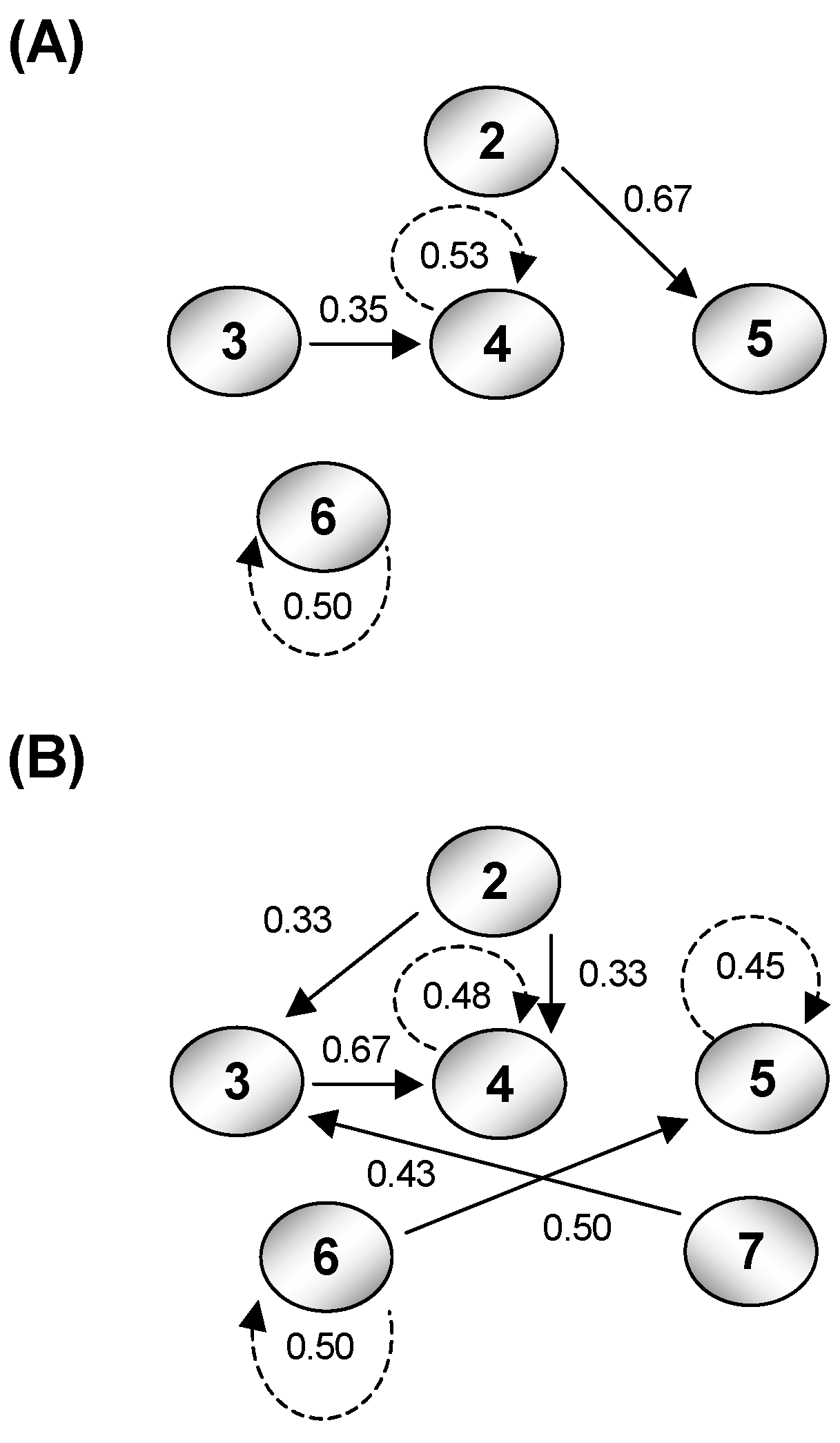
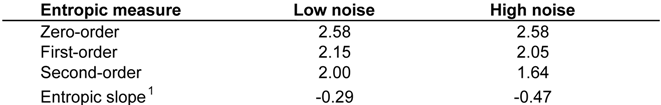
4. Calculating Effects of Boat Noise on the Channel Capacity of Glacier Bay
5. Comparing Channel Capacity with Humpback Whale Signal Transmission Rate
6. Conclusions
Acknowledgments
References
- Richardson, J. W. Marine Mammals and Noise; Academic Press: New York, NY, 1998. [Google Scholar]
- Baker, C. S.; Herman, L. M. Behavioral Responses of Summering Humpback Whales to Vessel Traffic: Experimental and Opportunistic Observations; U.S. National Park Service, Alaska Regional Office: Anchorage, AK, 1989; p. 50. [Google Scholar]
- Borggaard, D.; Lien, J.; Stevick, P. Assessing the Effects of Industrial Activity on Large Cetaceans in Trinity Bay, Newfoundland (1992-1995). Aquatic Mammals 1999, 25, 149–161. [Google Scholar]
- Cerchio, S. Cultural Evolution and Geographic Variation in the Songs of Humpback Whales in the Eastern North Pacific; University of California: Santa Cruz, CA, 1993. [Google Scholar]
- Cerchio, S.; Jacobsen, J. K.; Norris, T.F. Temporal and geographical variation in songs of humpback whales, Megaptera novaeangliae: Synchronous Change in Hawaiian and Mexican Breeding Assemblages. Animal Behaviour 2001, 62, 313–329. [Google Scholar] [CrossRef]
- Frankel, A. S.; Clark, C. W.; Herman, L. M.; Gabriele, C. M. Spatial distribution, habitat utilization, and social interactions of humpback whales, Megaptera Novaeangliae, Off Hawai'i, Determined Using Acoustic and Visual Techniques. Canadian Journal of Zoology 1995, 73, 1134–1146. [Google Scholar] [CrossRef]
- Hafner, G. W.; Hamilton, C. L.; Steiner, W. W.; Thompson, T. J.; Winn, H. E. Signature Information in the Song of the Humpback Whale Megaptera-Novaeangliae. Journal of the Acoustical Society of America 1979, 66, 1–6. [Google Scholar] [CrossRef]
- Helweg, D. A.; Cato, D. H.; Jenkins, P. F.; Garrigue, C.; McCauley, R. D. Geographic Variation in South Pacific Humpback Whale Songs. Behaviour 1998, 135, 1–27. [Google Scholar] [CrossRef]
- Helweg, D. A.; Herman, L. M.; Yamamoto, S.; Forestelsl, P. H. Comparison of Songs of Humpback Whales Megaptera-Novaeangliae Recorded in Japan Hawaii and Mexico During the Winter of 1989. Scientific Reports of Cetacean Research 1990, 1–20. [Google Scholar]
- Payne, R. S.; McVay, S. Songs of Humpback Whales. Science 1971, 173, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Baker, C. S. The Population Structure and Social Organization of Humpback Whales (Megaptera novaeangliae) in the Central and Eastern North Pacific; University of Hawaii: Honolulu, HI, 1985. [Google Scholar]
- Cerchio, S. Bioacoustic Analysis of Humpback Whale Vocalizations Recorded off the Aleutian Islands, Alaska. In Aleutian Island Marine Mammal Survey 1994; Southwest Fisheries Science Center, NMFS, NOAA: Aleutian Islands, 1996. [Google Scholar]
- Cerchio, S.; Dahlheim, M. Variation in Feeding Vocalizations of Humpback Whales Megaptera Novaeangliae from Southeast Alaska. Bioacoustics 2001, 11, 277–295. [Google Scholar] [CrossRef]
- Thompson, P. O.; Cummings, W. C.; Ha, S. J. Sounds, Source Levels, and Associated Behavior of Humpback Whales, Southeast Alaska. Journal of the Acoustical Society of America 1986, 80, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.; Payne, R. Large Scale Changes Over 19 Years in Songs of Humpback Whales in Bermuda. Z. Tierpsychologie 1985, 68, 89–114. [Google Scholar] [CrossRef]
- Fristrup, K. M.; Hatch, L. T.; Clark, C. W. Variation in Humpback Whale (Megaptera Novaeangliae) Song Length in Relation to Low-Frequency Sound Broadcasts. Journal of the Acoustical Society of America 2003, 113, 3411–3424. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.J.O.; Biassoni, N.; Samuels, A.; Tyack, P.L. Whale Songs Lengthen in Response to Sonar. Nature 2000, 405, 903. [Google Scholar] [CrossRef] [PubMed]
- Norris, T. F. Effects of Boat Noise on the Singing Behavior of Humpabck Whales; San Jose State University: San Jose, CA, 1995. [Google Scholar]
- Rabin, L.; McCowan, B.; Hooper, S. L.; Owings, D. Anthropogenic Noise and its Effect on Animal Communication: An Interface Between Comparative Psychology and Conservation Biology. International Journal of Comparative Psychology 2003, 16, 172–192. [Google Scholar]
- Slabbekoorn, H.; Peet, M. Birds Sing at a Higher Pitch in Urban Noise. Nature 2003, 424, 267. [Google Scholar] [CrossRef] [PubMed]
- McCowan, B.; Hanser, S. F.; Doyle, L. R. Quantitative Tools for Comparing Animal Communication Systems: Information Theory Applied to Bottlenose Dolphin Whistle Repertoires. Animal Behaviour 1999, 57, 409–419. [Google Scholar] [CrossRef] [PubMed]
- McCowan, B.; Doyle, L. R.; Hanser, S. F. Using Information Theory to Assess the Diversity, Complexity and Development of Communicative Repertoires. Journal of Comparative Psychology 2002, 116, 166–172. [Google Scholar] [CrossRef] [PubMed]
- McCowan, B.; Doyle, L. R.; Hanser, S. F.; Kaufman, A.; Burgess, C. Detection and Estimation of Complexity in Nonhuman Animal Communication. In Animal Communications; MIT Press: Cambridge, MA, 2008. (in press) [Google Scholar]
- Shannon, C. E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urban, IL, 1949. [Google Scholar]
- Chatfield, C.; Lemon, R. Analyzing Sequences of Behavioral Events. Journal of Theoretical Biology 1970, 29, 427–445. [Google Scholar] [CrossRef]
- Pierce, J.R. An Introduction to Information Theory; Dover Publications: New York, 1980. [Google Scholar]
- McCowan, B. A New Quantitative Technique for Categorizing Whistles Using Simulated Signals and Whistles from Captive Bottlenose Dolphins (Delphindae Tursiops Truncatus). Ethology 1995, 100, 177–193. [Google Scholar] [CrossRef]
- McCowan, B.; Hooper, S. L. Individual Acoustic Variation in Belding's Ground Squirrel Alarm Chirps in the High Sierra Nevada. Journal of the Acoustical Society of America 2002, 111, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- McCowan, B.; Reiss, D. The Fallacy of 'Signature Whistles' in Bottlenose Dolphins: a Comparative Perspective of Signature Information' in Animal Vocalizations. Animal Behaviour 2001, 62, 1151–1162. [Google Scholar] [CrossRef]
- Law, A. M.; Kelton, W. D. Simulation Modeling and Analysis; McGraw-Hill: Boston, MA, 2000. [Google Scholar]
- Efron, B.; Tibshirani, R. J. An Introduction to the Bootstrap; CRC Press: Boca Raton, FL, 1998. [Google Scholar]
- Sharpe, F.A. Social Foraging of the Southeast Alaskan Humpback Whale, Megaptera novaeangliae. Ph.D. Thesis, Simon Fraser University, Burnaby, British Columbia, Canada.
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Doyle, L.R.; McCowan, B.; Hanser, S.F.; Chyba, C.; Bucci, T.; Blue, J.E. Applicability of Information Theory to the Quantification of Responses to Anthropogenic Noise by Southeast Alaskan Humpback Whales. Entropy 2008, 10, 33-46. https://doi.org/10.3390/entropy-e10020033
Doyle LR, McCowan B, Hanser SF, Chyba C, Bucci T, Blue JE. Applicability of Information Theory to the Quantification of Responses to Anthropogenic Noise by Southeast Alaskan Humpback Whales. Entropy. 2008; 10(2):33-46. https://doi.org/10.3390/entropy-e10020033
Chicago/Turabian StyleDoyle, Laurance R., Brenda McCowan, Sean F. Hanser, Christopher Chyba, Taylor Bucci, and J. Ellen Blue. 2008. "Applicability of Information Theory to the Quantification of Responses to Anthropogenic Noise by Southeast Alaskan Humpback Whales" Entropy 10, no. 2: 33-46. https://doi.org/10.3390/entropy-e10020033




