Investigation of the Intra- and Inter-Limb Muscle Coordination of Hands-and-Knees Crawling in Human Adults by Means of Muscle Synergy Analysis
Abstract
:1. Introduction
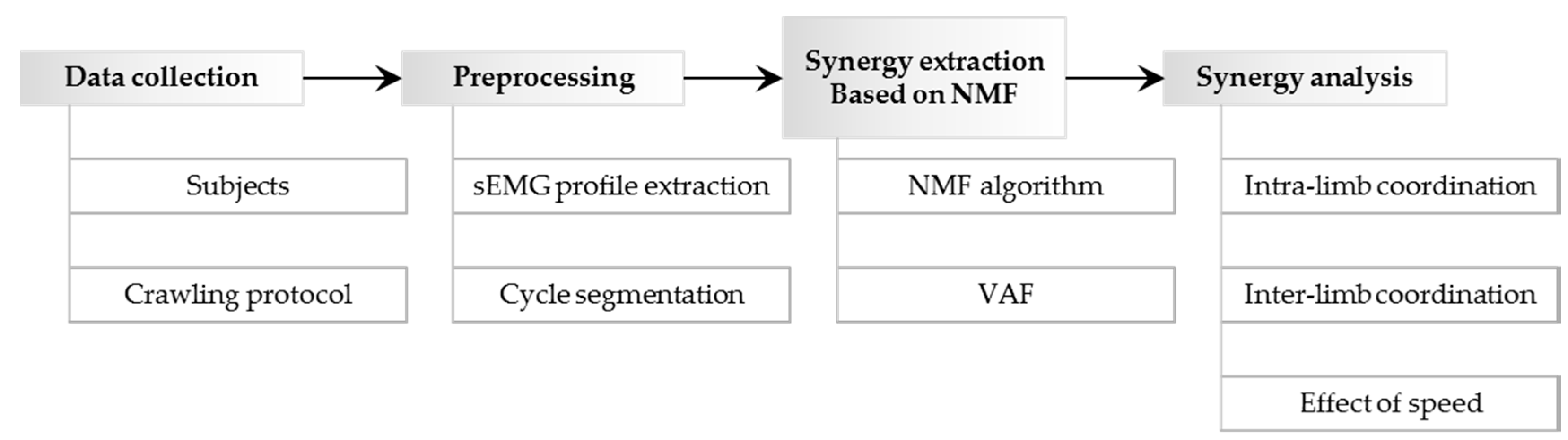
2. Materials and Methods
- Subjects were recruited to complete a number of hands-and-knees crawling experiments under different crawling speeds;
- Surface EMG signals were recorded from 32 main functional muscles of the subjects during the crawling task;
- Muscle synergies of four limbs were extracted respectively using the nonnegative matrix factorization (NMF) algorithm based on the sEMG data;
- Muscle synergies analysis under different conditions was conducted to explore the underlying control mechanism of human hands-and-knees crawling.
2.1. Subjects and Crawling Protocol
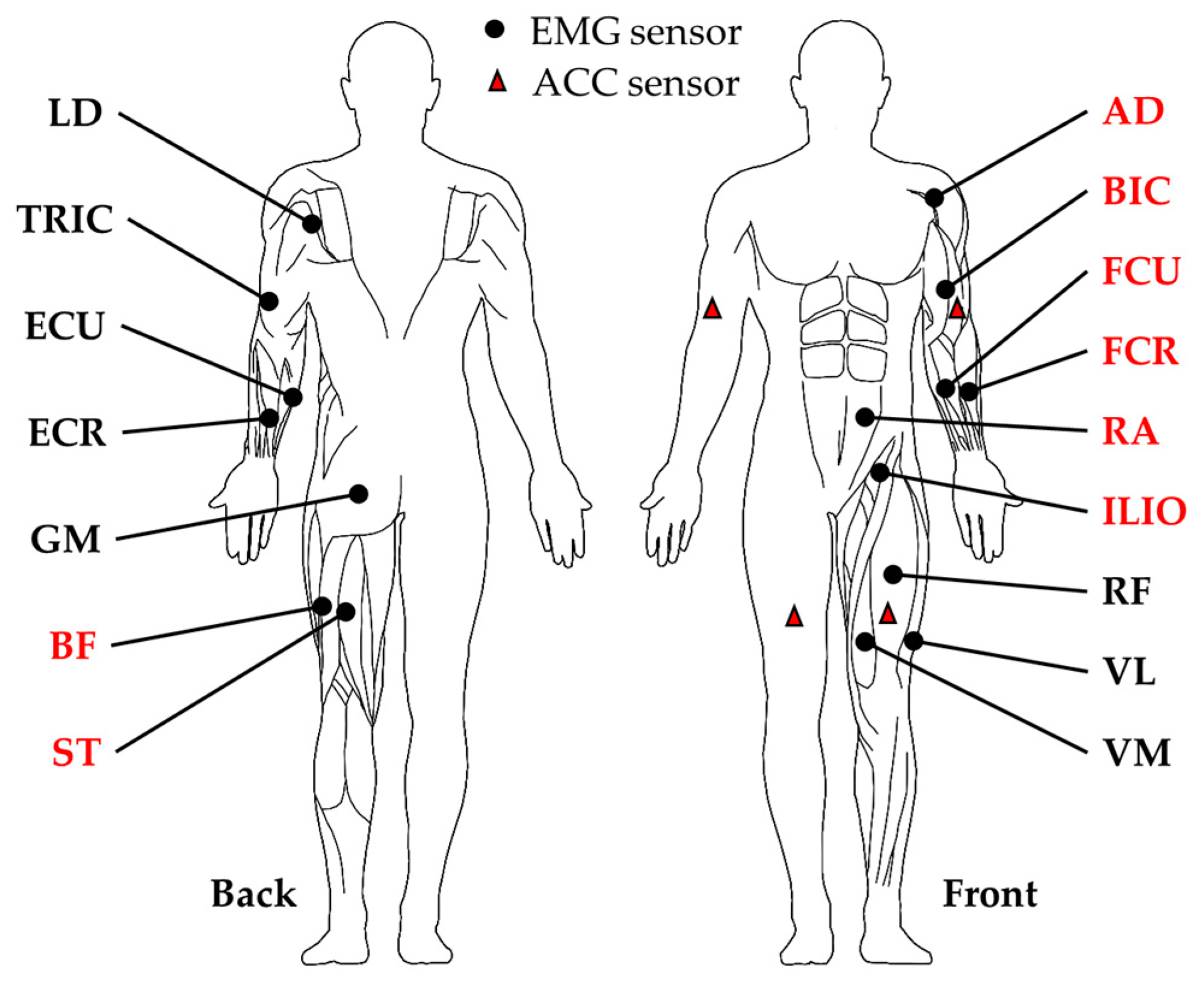
2.2. Crawling Data Collection
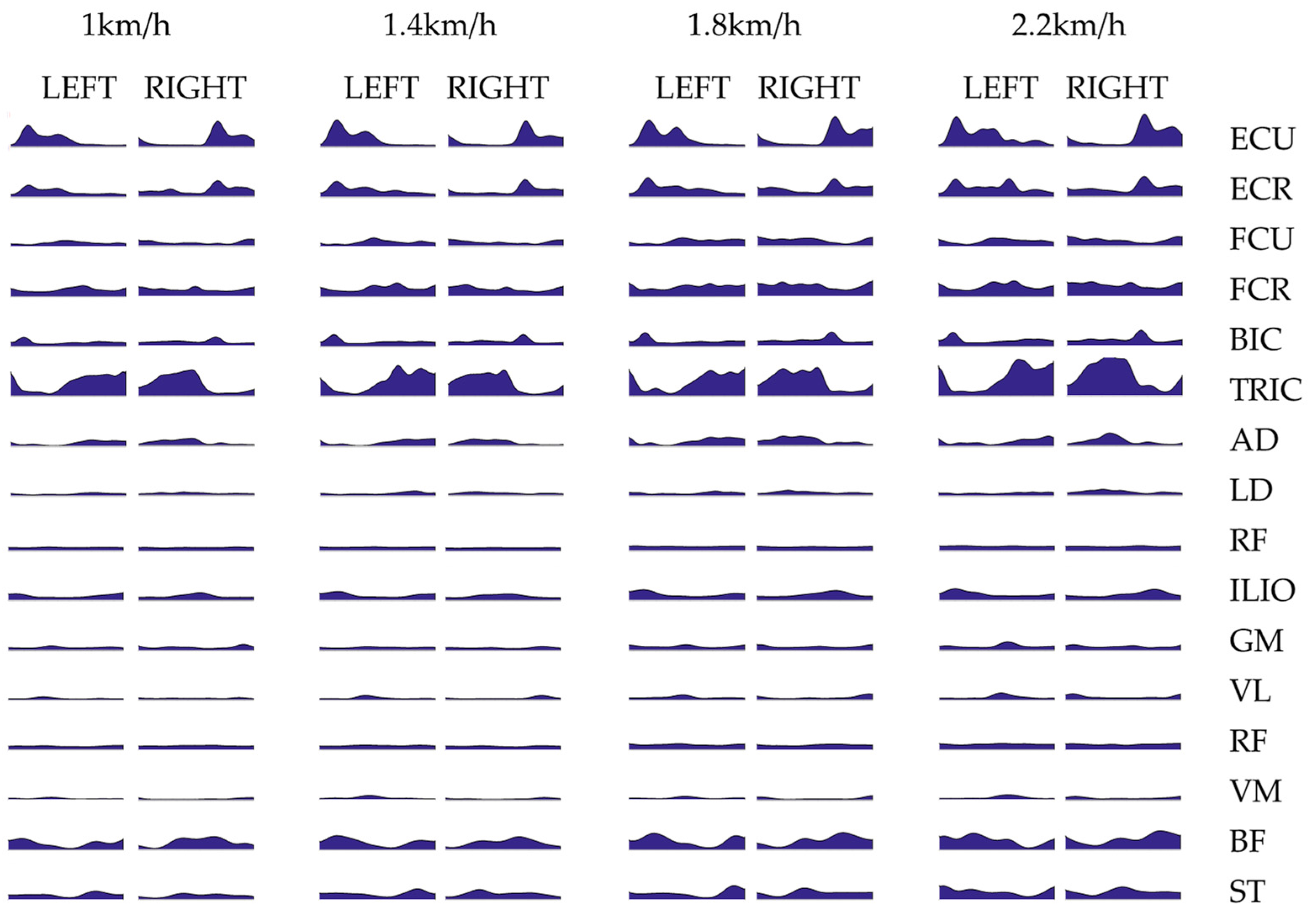
2.3. Surface EMG Profile Extraction and Cycle Segmentation
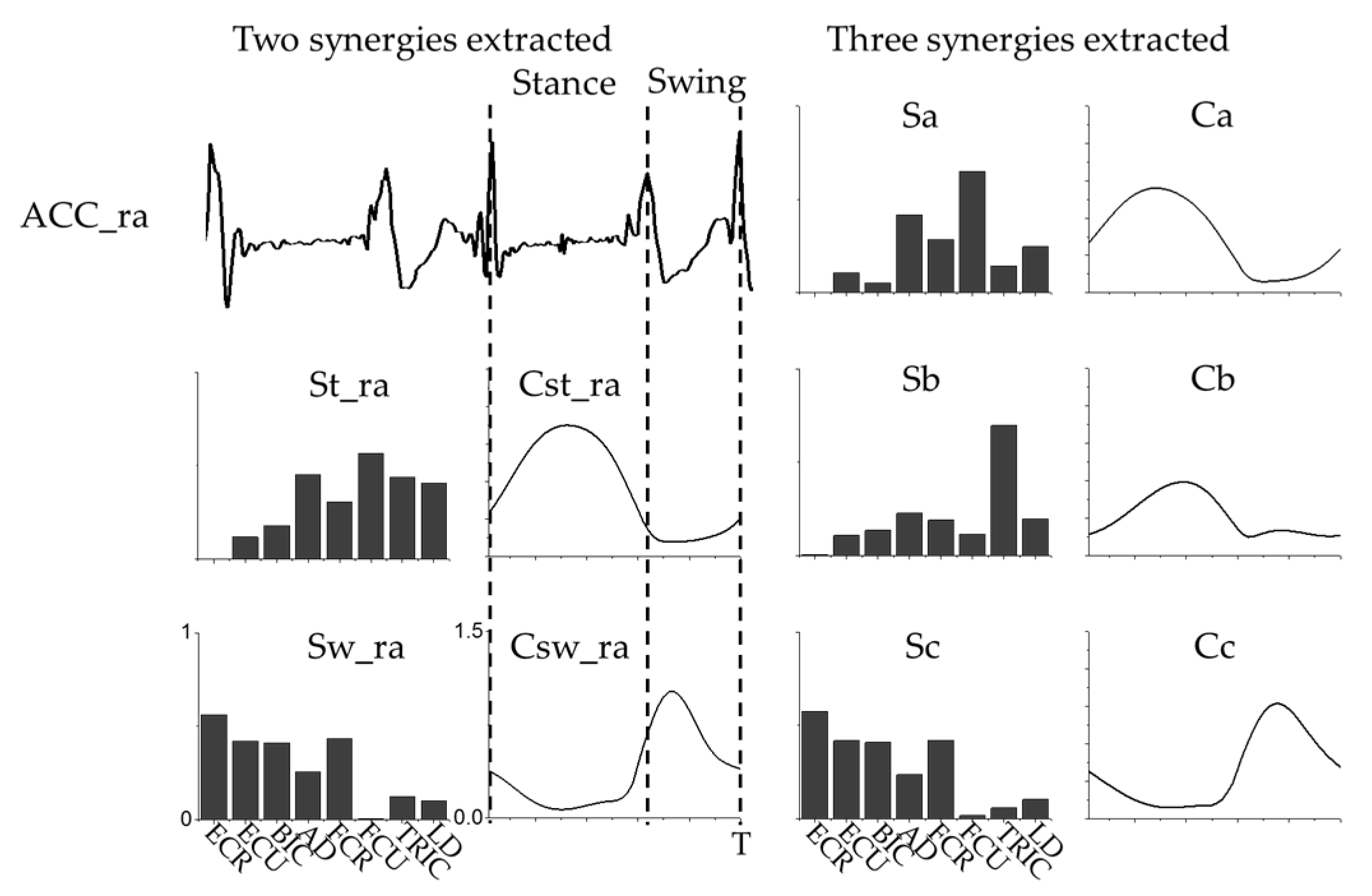
2.4. Nonnegative Matrix Factorization Based Muscle Synergy Extraction
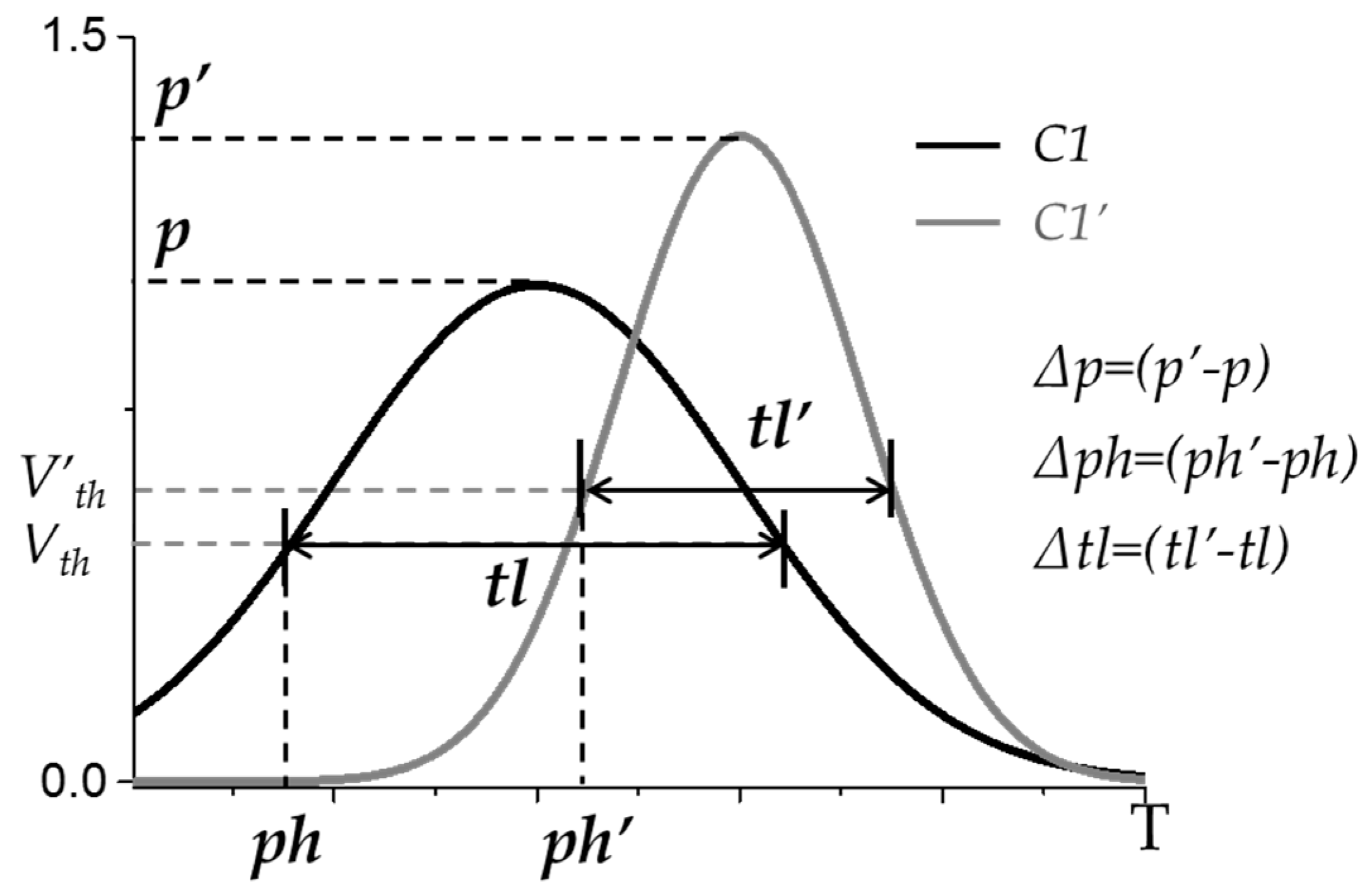
2.5. Similarities between Muscle Synergy Structures and between Recruitment Patterns
2.6. Intra-Limb Muscle Coordination Analysis
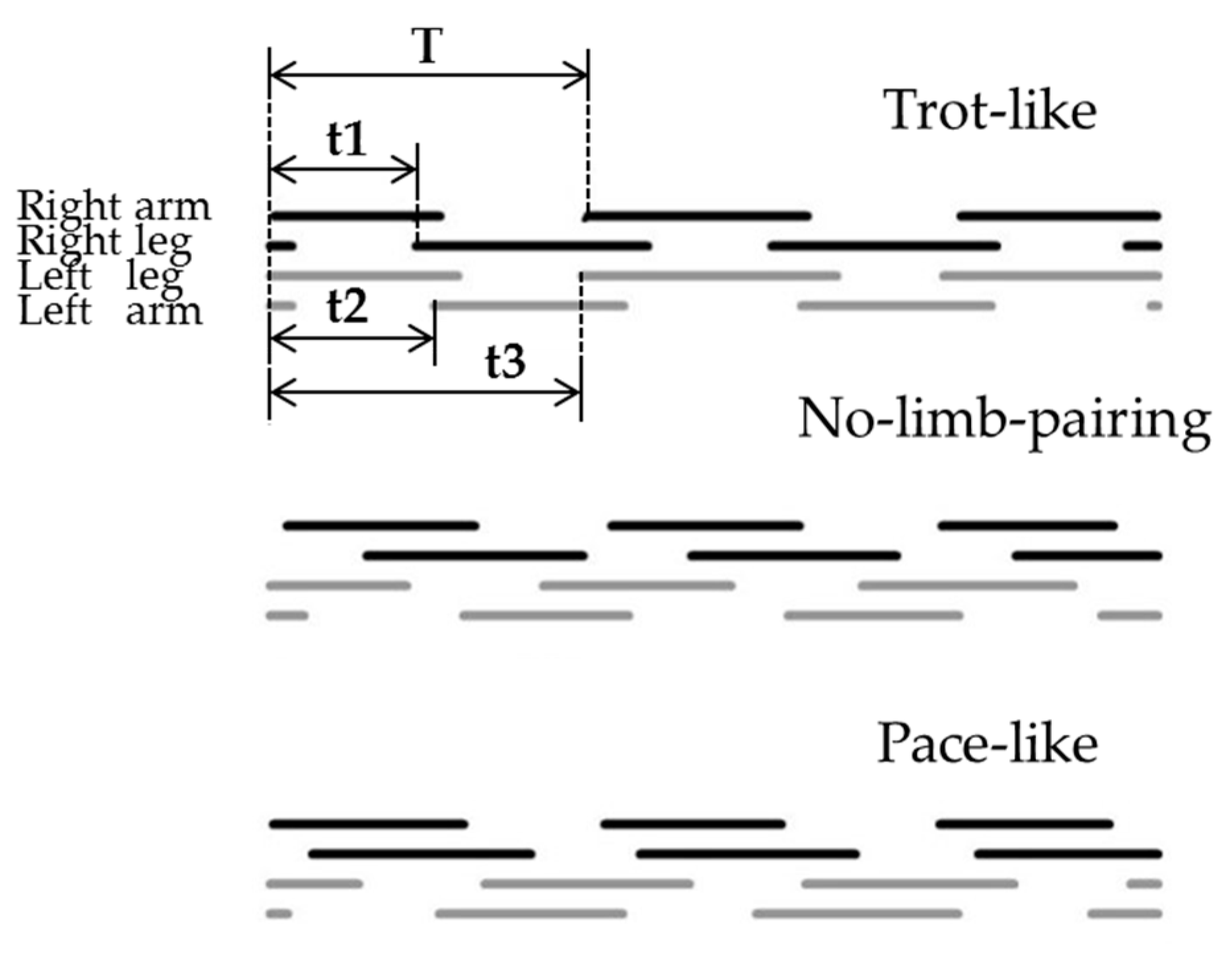
2.7. Inter-Limb Coordination Analysis
3. Results
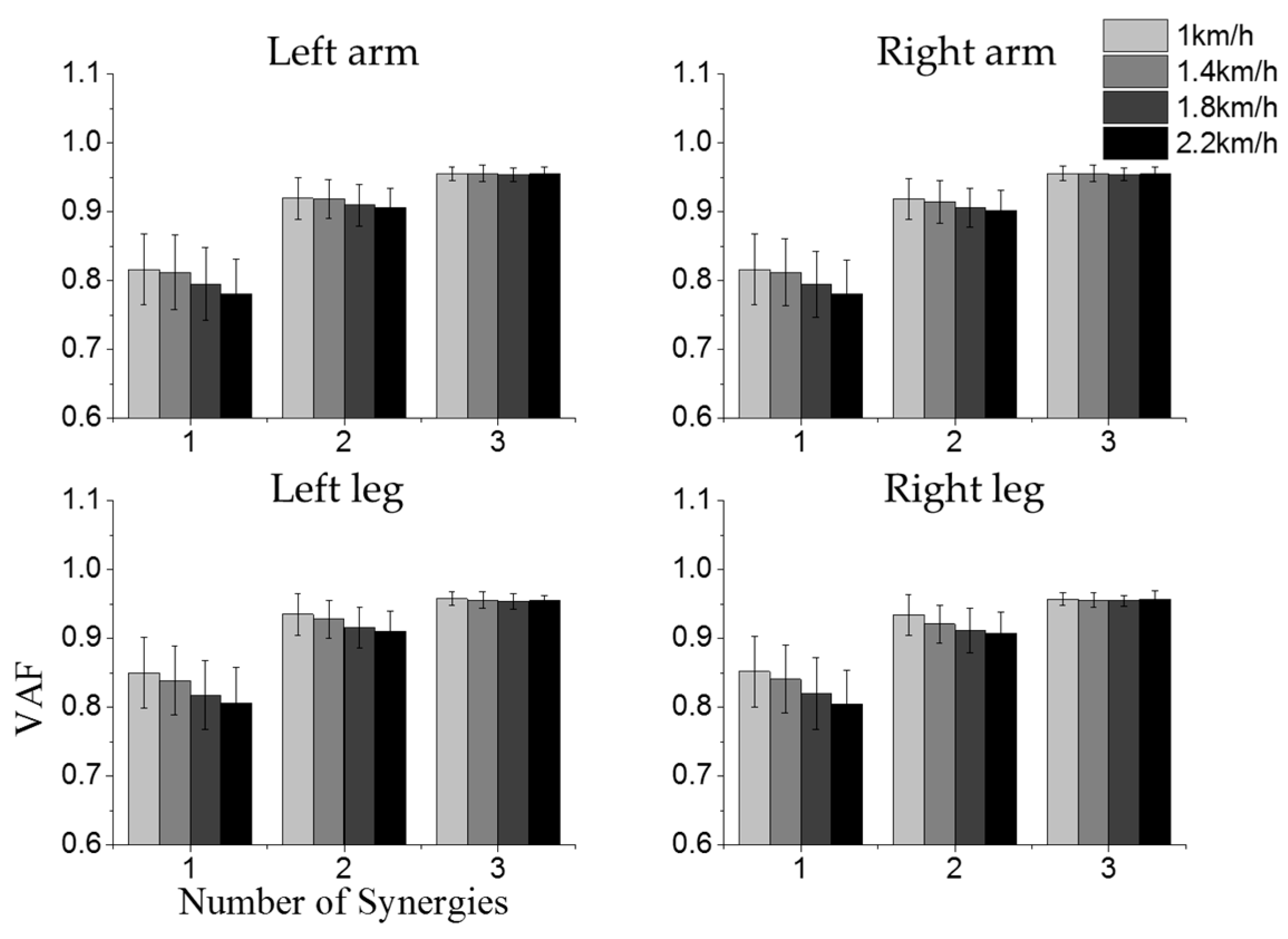
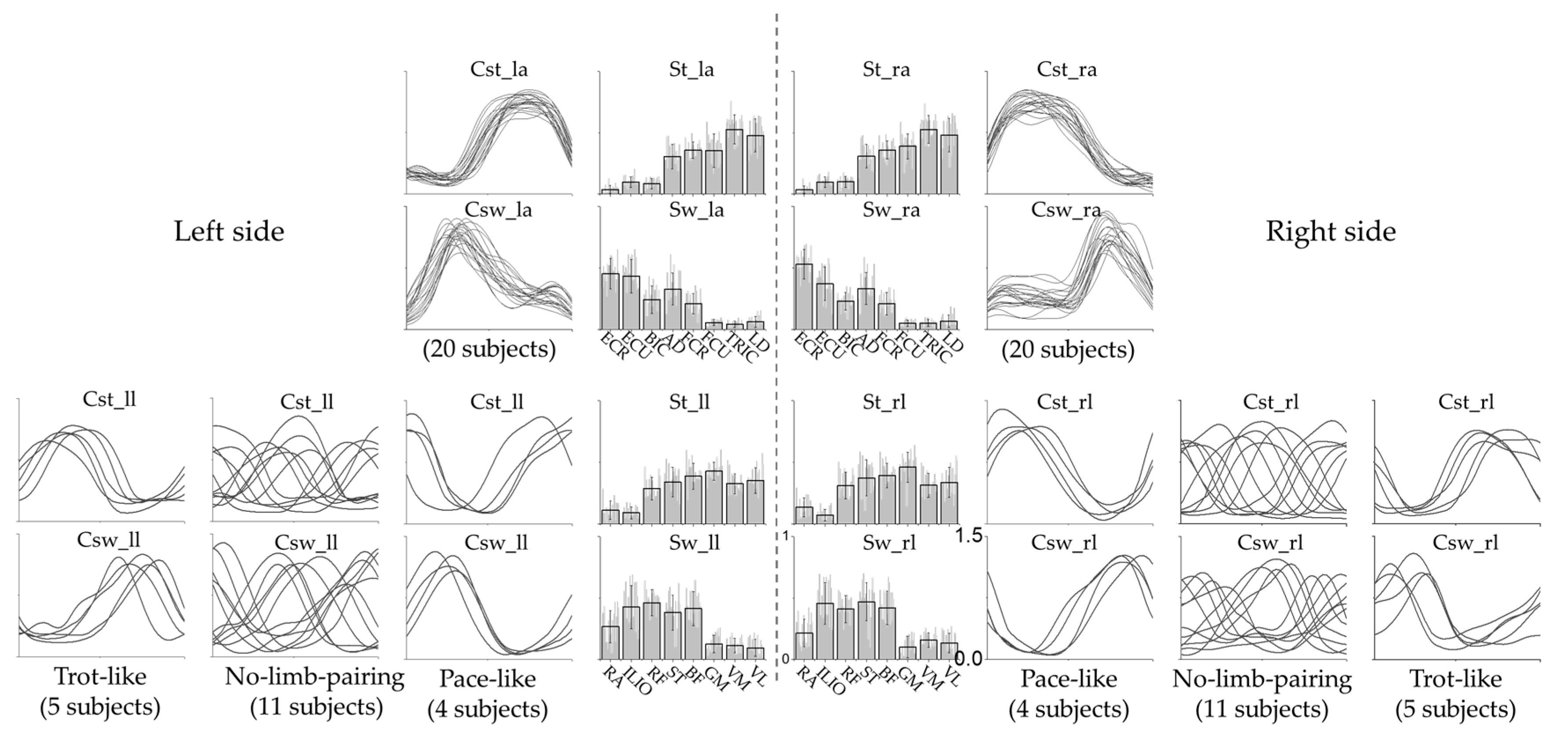
3.1. Intra-Limb Muscle Coordination
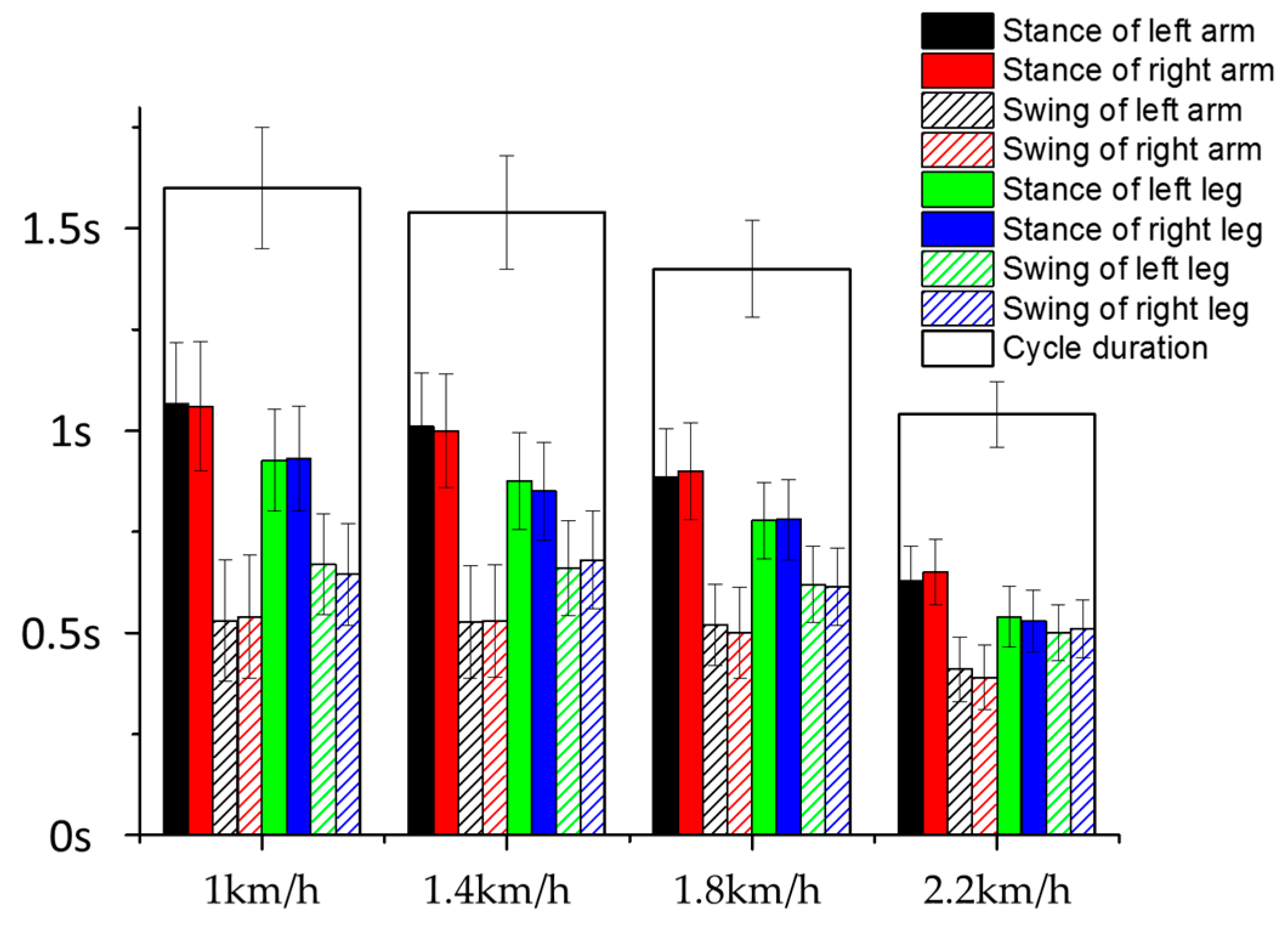
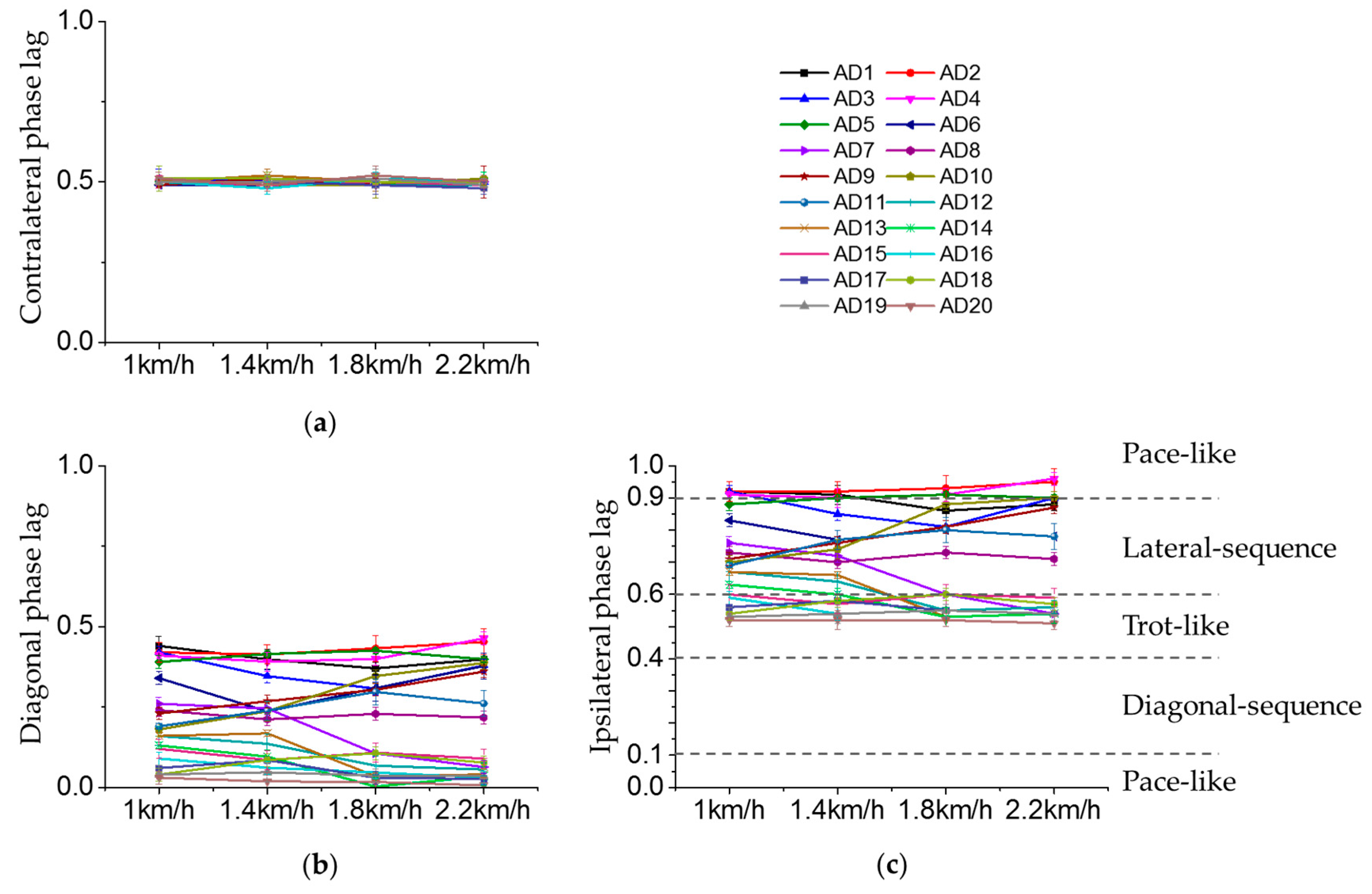
3.2. Inter-Limb Coordination
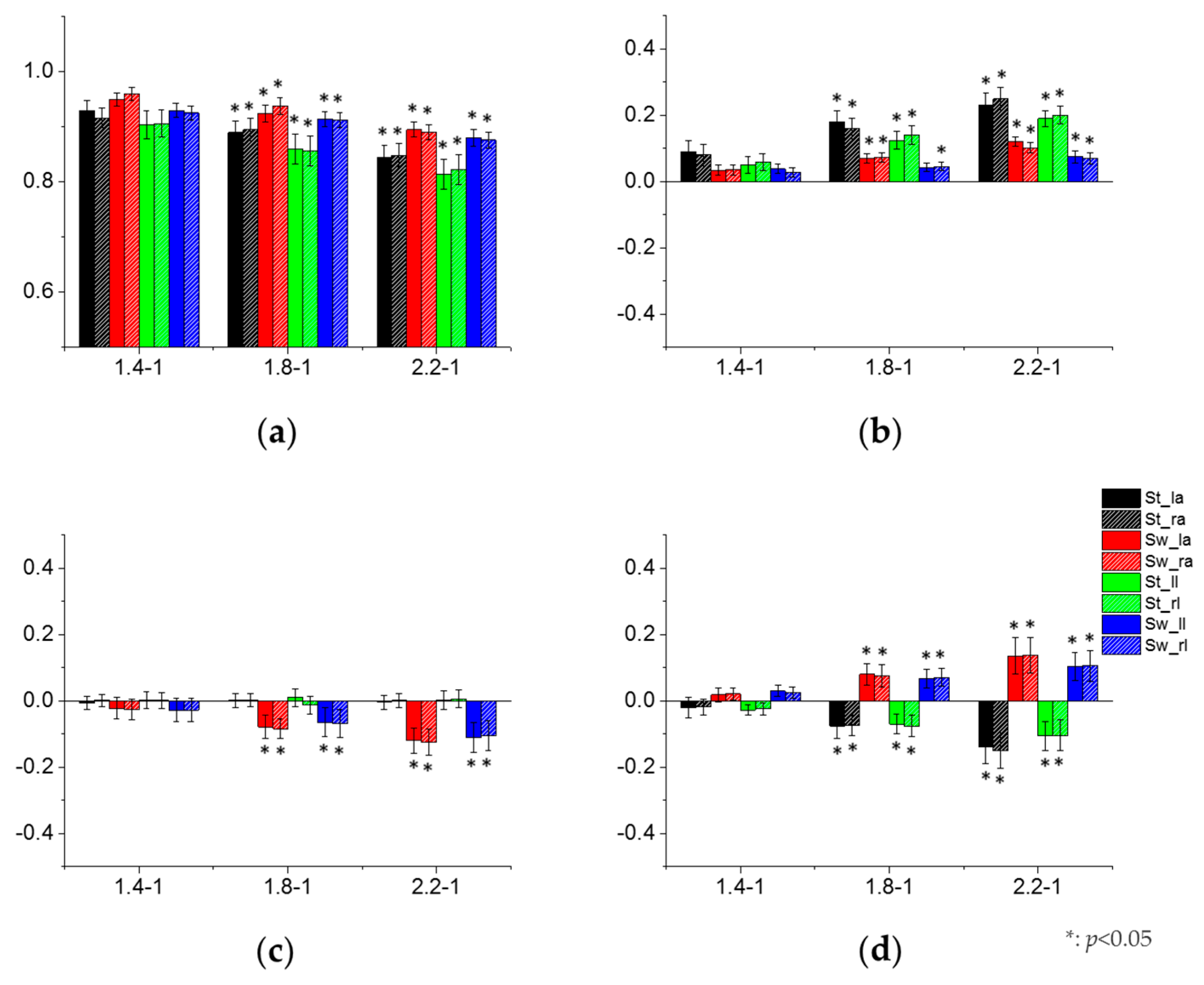
3.3. Effect of Crawling Speed on Intra- and Inter-Limb Coordination
4. Discussion
4.1. The Possible CPG Model for Hands-and-Knees Crawling
4.2. Control Strategies of CNS to Crawling Speed
4.3. Limitations and Future Work
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McGraw, M.B. Neural maturation as exemplified in the changing reactions of the infant to pin prick. Child Dev. 1941, 12, 31–42. [Google Scholar] [CrossRef]
- Adolph, K.E.; Vereijken, B.; Denny, M.A. Learning to crawl. Child Dev. 1998, 69, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M. Symmetrical gaits of primates. Am. J. Phys. Anthropol. 1967, 26, 119. [Google Scholar] [CrossRef]
- Hildebrand, M. Symmetrical gait of horses. Science 1965, 150, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M. Analysis of tetrapod gaits: General considerations and symmetrical gaits. Neural Control Locomot. 1976, 18, 203–206. [Google Scholar] [CrossRef]
- Patrick, S.K.; Noah, J.A.; Yang, J.F. Interlimb coordination in human crawling reveals similarities in development and neural control with quadrupeds. J. Neurophysiol. 2009, 101, 603–613. [Google Scholar] [CrossRef] [PubMed]
- MacLellan, M.J.; Ivanenko, Y.P.; Catavitello, G.; La Scaleia, V.; Lacquaniti, F. Coupling of upper and lower limb pattern generators during human crawling at different arm/leg speed combinations. Exp. Brain Res. 2013, 225, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Tan, U.; Derawi, M.O.; Bours, P.; Holien, K. Quadrupedal locomotor characteristics of uner tan syndrome cases, healthy humans, and nonhuman primates in evolutionary perspectives improved cycle detection for accelerometer based gait authentication. In Proceedings of the 2010 Sixth International Conference on Intelligent Information Hiding and Multimedia Signal Processing, Darmstadt, Germany, 15–17 October 2010. [Google Scholar]
- Righetti, L.; Nylen, A.; Rosander, K.; Iispeert, A.J. Kinematic and gait similarities between crawling human infants and other quadruped mammals. Front. Neurol. 2015, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Ting, L.H.; McKay, J.L. Neuromechanics of muscle synergies for posture and movement. Curr. Opin. Neurobiol. 2007, 17, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Danner, S.M.; Hofstoetter, U.S.; Freundl, B.; Binder, H.; Mayr, W.; Rattay, F.; Minassian, K. Human spinal locomotor control is based on flexibly organized burst generators. Brain 2015, 138, 577–588. [Google Scholar] [CrossRef] [PubMed]
- D’Avella, A.; Saltiel, P.; Bizzi, E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat. Neurosci. 2003, 6, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Ting, L.H.; Macpherson, J.M. A limited set of muscle synergies for force control during a postural task. J. Neurophysiol. 2005, 93, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.J.; Eskinazi, I.; Jackson, J.N.; Rao, A.V.; Patten, C.; Fregly, B.J. Muscle synergies facilitate computational prediction of subject-specific walking motions. Front. Bioeng. Biotechnol. 2016, 4, 77. [Google Scholar] [CrossRef] [PubMed]
- Chvatal, S.A.; Ting, L.H. Voluntary and reactive recruitment of locomotor muscle synergies during perturbed walking. J. Neurosci. 2012, 32, 12237–12250. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Five basic muscle activation patterns account for muscle activity during human locomotion. J. Physiol. 2004, 556, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Dominici, N.; Ivanenko, Y.P.; Cappellini, G.; D’Avella, A.; Mondi, V.; Cicchese, M.; Fabiano, A.; Silei, T.; Di Paolo, A.; Giannini, C.; et al. Locomotor Primitives in Newborn Babies and Their Development. Science 2011, 334, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, F.; Cao, S.; Zhang, X.; Wu, D.; Chen, X. Muscle synergy analysis in children with cerebral palsy. J. Neural Eng. 2015, 12, 46017. [Google Scholar] [CrossRef] [PubMed]
- Chia, B.N.; Pedrocchi, A.; Nardone, A.; Schieppati, M.; Baccinelli, W.; Monticone, M.; Ferrigno, G.; Ferrante, S. Tuning of muscle synergies during walking along rectilinear and curvilinear trajectories in humans. Ann. Biomed. Eng. 2017, 45, 1204–1218. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures-Journal of Electromyography and Kinesiology. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Boettcher, C.E.; Ginn, K.A.; Cathers, I. Standard maximum isometric voluntary contraction tests for normalizing shoulder muscle EMG. J. Orthop. Res. 2008, 26, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Barr, A.E.; Goldsheyder, D.; Ozkaya, N.; Nordin, M. Testing apparatus and experimental procedure for position specific normalization of electromyographic measurements of distal upper extremity musculature. Clin. Biomech. 2001, 16, 576–585. [Google Scholar] [CrossRef]
- Veragarcia, F.J.; Moreside, J.M.; Mcgill, S.M. MVC techniques to normalize trunk muscle EMG in healthy women. J. Electromyogr. Kinesiol. 2010, 20, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Rouffet, D.M.; Hautier, C.A. EMG normalization to study muscle activation in cycling. J. Electromyogr. Kinesiol. 2008, 18, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, S.; Pollard, J.; Porter, W.L. Locomotion in restricted space: Kinematic and electromyographic analysis of stoopwalking and crawling. Gait Posture 2011, 33, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.D.; Seung, H.S. Learning the parts of objects by non-negative matrix factorization. Nature 1999, 401, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, X.; Cao, S.; Zhang, X. Muscle-tendon units localization and activation level analysis based on high-density surface EMG array and NMF algorithm. J. Neural Eng. 2016, 13, 066001. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A.; Yack, H.J. EMG profiles during normal human walking: Stride-to-stride and inter-subject variability. Electroencephalogr. Clin. Neurophysiol. 1987, 67, 402–411. [Google Scholar] [CrossRef]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J. Neurophysiol. 2010, 103, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Spinal cord maps of spatiotemporal alpha-motoneuron activation in humans walking at different speeds. J. Neurophysiol. 2006, 95, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Maclellan, M.J.; Ivanenko, Y.P.; Cappellini, G.; Sylos, L.F.; Lacquaniti, F. Features of hand-foot crawling behavior in human adults. J. Neurophysiol. 2012, 107, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, A.B.; Winter, D.A.; Marteniuk, R.G. Is there a ‘normal’ profile of EMG activity in gait? Med. Biol. Eng. Comput. 1986, 24, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V.; Fouad, K.; Bastiaanse, C.M. Neuronal coordination of arm and leg movements during human locomotion. Eur. J. Neurosci. 2001, 14, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Derawi, M.O.; Bours, P.; Holien, K. Improved cycle detection for accelerometer based gait authentication. In Proceedings of the Sixth International Conference on Intelligent Information Hiding and Multimedia Signal Processing (IIH-MSP), Darmstadt, Germany, 15–17 October 2010; pp. 312–317. [Google Scholar] [CrossRef]
- Takeda, R.; Tadano, S.; Todoh, M.; Morikawa, M.; Nakayasu, M.; Yoshinari, S. Gait analysis using gravitational acceleration measured by wearable sensors. J. Biomech. 2009, 42, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.; Tong, K. The reliability of using accelerometer and gyroscope for gait event identification on persons with dropped foot. Gait Posture 2008, 27, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.G. The intrinsic factors in the act of progression in the mammal. Proc. R. Soc. Lond. B Biol. Sci. 1911, 84, 308–319. [Google Scholar] [CrossRef]
- Graham, B.T. On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. J. Physiol. 1914, 48, 18–46. [Google Scholar] [CrossRef]
- Grillner, S. Control of locomotion in bipeds, tetrapods, and fish. Compr. Physiol. 2011. [Google Scholar] [CrossRef]
- Rybak, I.A.; Shevtsova, N.A.; Lafreniere-Roula, M.; McCrea, D.A. Modelling spinal circuitry involved in locomotor pattern generation: Insights from deletions during fictive locomotion. J. Physiol. 2006, 577, 617–639. [Google Scholar] [CrossRef] [PubMed]
- Hagglund, M.; Dougherty, K.J.; Borgius, L.; Itohara, S.; Iwasato, T.; Kiehn, O. Optogenetic dissection reveals multiple rhythmogenic modules underlying locomotion. Proc. Natl. Acad. Sci. USA 2013, 110, 11589–11594. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S.; El Manira, A. The intrinsic operation of the networks that make us locomote. Curr. Opin. Neurobiol. 2015, 31, 244–249. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.L.; Dougherty, K.J. Peeling back the layers of locomotor control in the spinal cord. Curr. Opin. Neurobiol. 2015, 33, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, S. Neural control of stereotypic limb movements. Compr. Physiol. 1996. [Google Scholar] [CrossRef]
- Kiehn, O. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 2006, 29, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S.; Zangger, P. On the central generation of locomotion in the low spinal cat. Exp. Brain Res. 1979, 34, 241–261. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.S.; Bartos, I.; Márka, Z.; Akay, T.; Márka, S.; Mann, R.S. Quantification of gait parameters in freely walking rodents. BMC Biol. 2015, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Bellardita, C.; Kiehn, O. Phenotypic characterization of speed-associated gait changes in mice reveals modular organization of locomotor networks. Curr. Biol. 2015, 25, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Danner, S.M.; Wilshin, S.D.; Shevtsova, N.A.; Rybak, I.A. Central control of interlimb coordination and speed-dependent gait expression in quadrupeds. J. Physiol. 2016, 594, 6947–6967. [Google Scholar] [CrossRef] [PubMed]
- Monaco, V.; Ghionzoli, A.; Micera, S. Age-related modifications of muscle synergies and spinal cord activity during locomotion. J. Neurophysiol. 2010, 104, 2092–2102. [Google Scholar] [CrossRef] [PubMed]
| Pattern | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pace-like | 0.89 ± 0.04 | 0.90 ± 0.03 | 0.87 ± 0.06 | 0.88 ± 0.05 | 0.83 ± 0.10 | 0.82 ± 0.10 | 0.80 ± 0.11 | 0.81 ± 0.13 |
| No-limb-pairing | 0.87 ± 0.05 | 0.89 ± 0.06 | 0.85 ± 0.09 | 0.87 ± 0.07 | 0.12 ± 0.74 | 0.14 ± 0.71 | 0.19 ± 0.66 | 0.21 ± 0.62 |
| Trot-like | 0.92 ± 0.04 | 0.93 ± 0.03 | 0.86 ± 0.05 | 0.88 ± 0.04 | 0.82 ± 0.11 | 0.80 ± 0.13 | 0.76 ± 0.19 | 0.78 ± 0.17 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Niu, X.; Wu, D.; Yu, Y.; Zhang, X. Investigation of the Intra- and Inter-Limb Muscle Coordination of Hands-and-Knees Crawling in Human Adults by Means of Muscle Synergy Analysis. Entropy 2017, 19, 229. https://doi.org/10.3390/e19050229
Chen X, Niu X, Wu D, Yu Y, Zhang X. Investigation of the Intra- and Inter-Limb Muscle Coordination of Hands-and-Knees Crawling in Human Adults by Means of Muscle Synergy Analysis. Entropy. 2017; 19(5):229. https://doi.org/10.3390/e19050229
Chicago/Turabian StyleChen, Xiang, Xiaocong Niu, De Wu, Yi Yu, and Xu Zhang. 2017. "Investigation of the Intra- and Inter-Limb Muscle Coordination of Hands-and-Knees Crawling in Human Adults by Means of Muscle Synergy Analysis" Entropy 19, no. 5: 229. https://doi.org/10.3390/e19050229






