Use of Mutual Information and Transfer Entropy to Assess Interaction between Parasympathetic and Sympathetic Activities of Nervous System from HRV
Abstract
:1. Introduction
2. Methods
2.1. Datasets
2.2. RR Intervals Preprocessing
2.3. Frequency Domain and Interaction Analysis of HRV
2.3.1. Frequency Domain Features
- LF power in normalized units (nuLF) was calculated by the power in LF in proportion to total power minus the power in VLF. The formula is as follows [32]:
- HF power in normalized units (nuHF) was calculated by the power in HF in proportion to total power minus the power in VLF. The formula is as follows [32]:
- LF/HF ratio was calculated by the power in LF in proportion to the power in HF. The formula is as follows [32]:
2.3.2. Interaction Features
- MI was calculated to estimate the share information between parasympathetic and sympathetic of the autonomic nervous system. The formula is as follows [33]:For two and , where N is the length of time series.Time series X and Y were replaced by time series RRL and RRH, respectively. Then, Equation (4) can be expressed as followswhere RRL is the time signal without HF components, and RRH is the time signal without LF components. N is the length of the time signal, i and j are progressive counter. is the join probability distribution of RRL and RRH. and are the marginal probability distributions of RRL and RRH. If RRL and RRH are independent, then MI = 0. It is noteworthy that MI is symmetric under the information transformed between RRL and RRH. Therefore, it does not contain any directional information.
- TE was calculated to quantify coupling strength and directional information between parasympathetic and sympathetic of the autonomic nervous system. The formula is as follows [24]:where is the joint probabilities of RRL(i), RRI(i − 1) and RRH(i − 1). The classic approach of fixed bins is used to estimate the probability in Equation (6), which is to allocate data points to fixed, equally-spaced bins. The following step is to rearrange the time signal RRL and RRH by ranging from smallest value to largest value of them, obtaining and . Then, Equation (6) can be expressed as follows [24]:Using fixed bins, Equation (7) can be expressed as follows:where Q is the unified number of bin. a, b and c represent the index of bins along the along the and respectively, P is the total number of triplets of and . , and are the number of data points in the intersection of the one-dimensional bins indexed by their subscript, and the number of data points in bth bin in the dimension denoted by .
2.4. Simulated Data
2.5. Statistical Analysis
3. Results
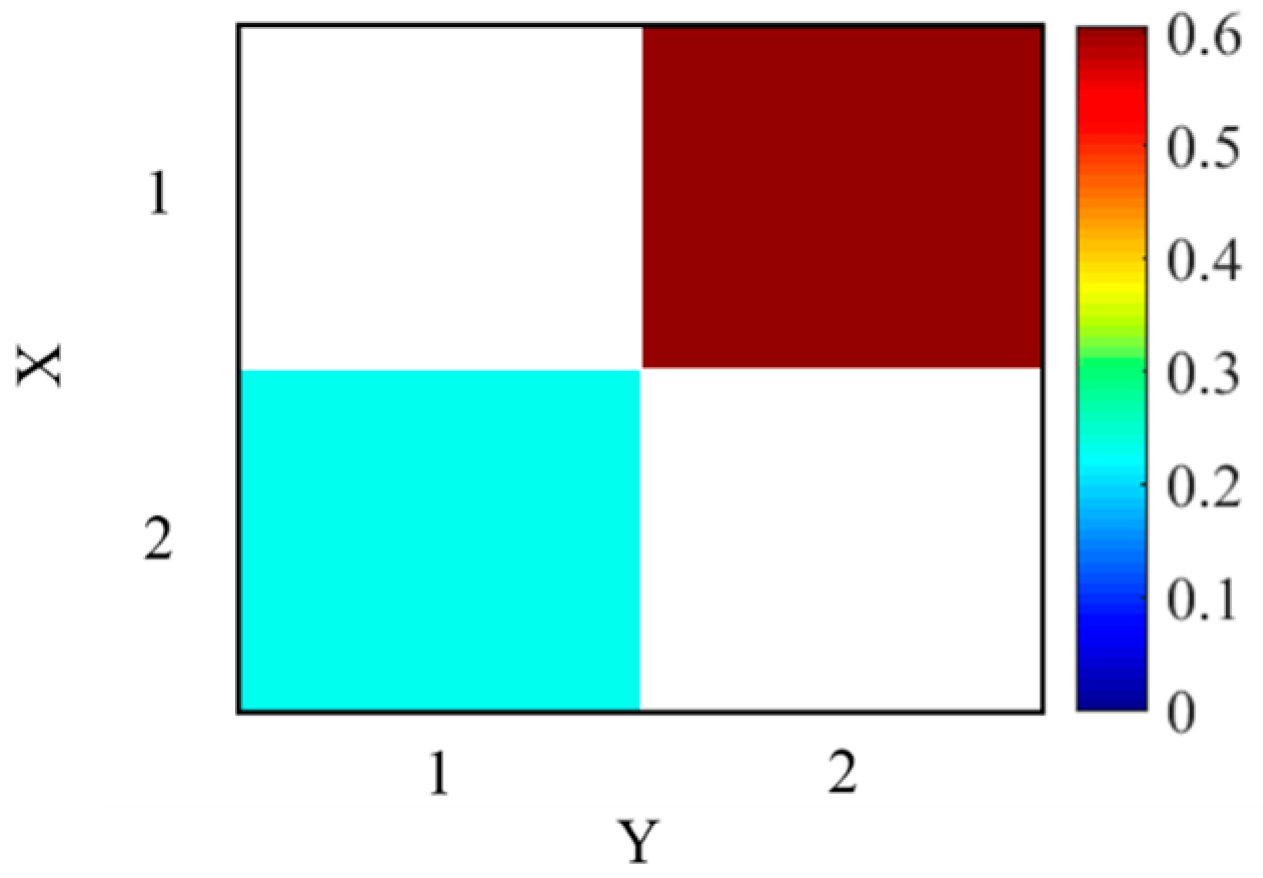
3.1. Simulated Experiment
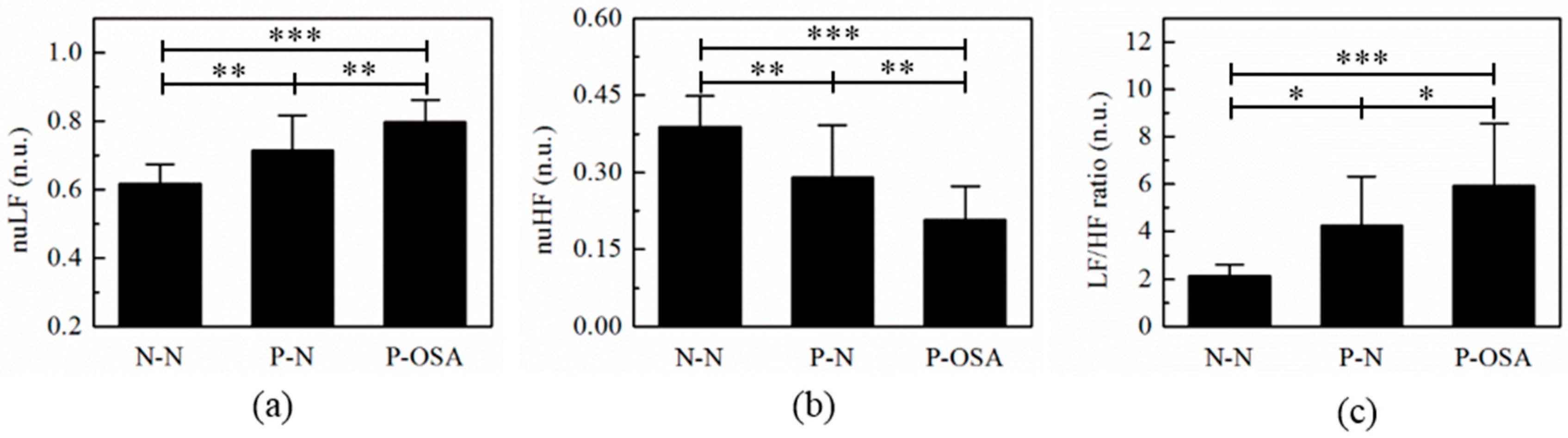
3.2. The Frequency Domain Features Analysis
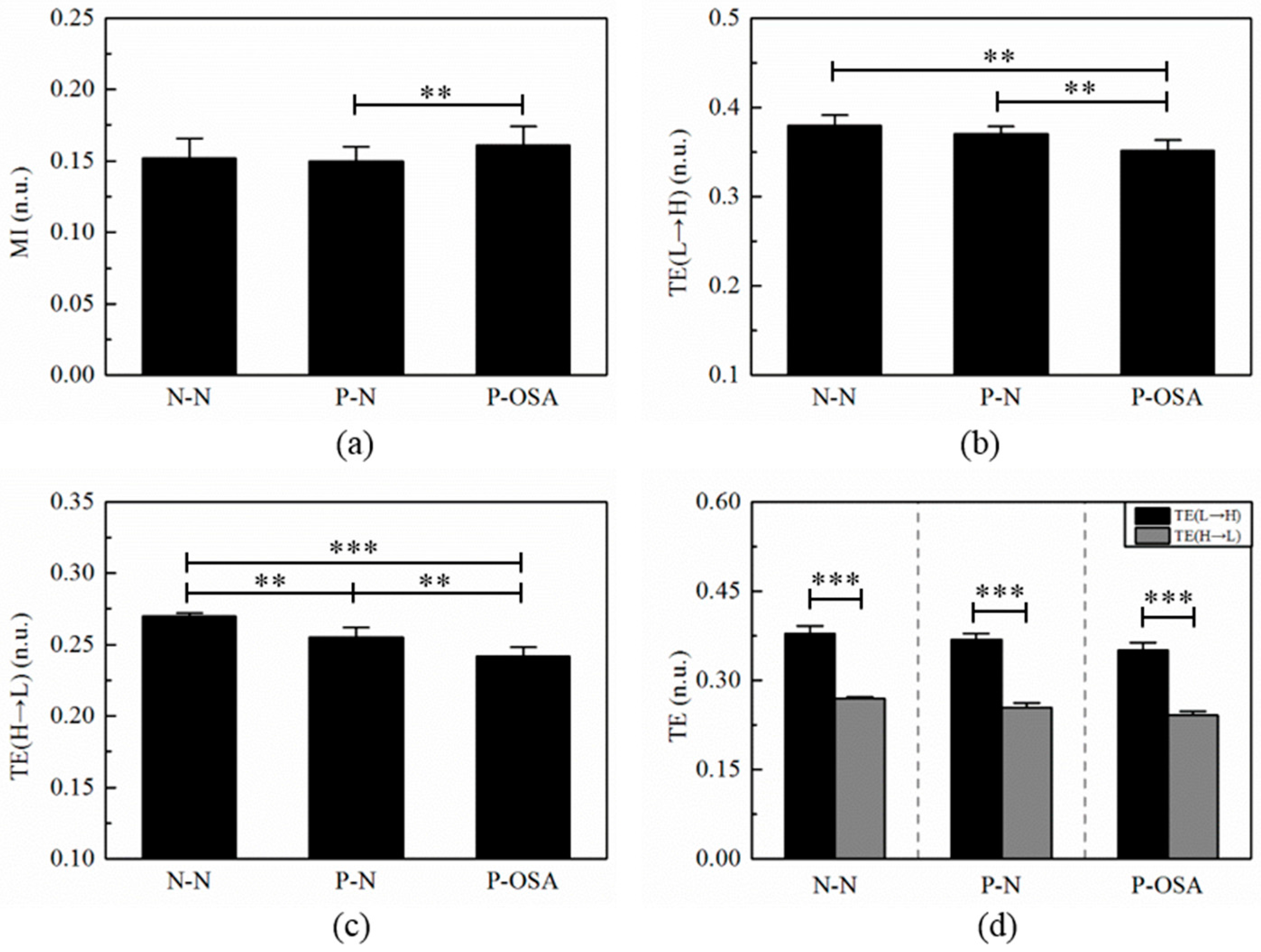
3.3. The Interaction Features Analysis
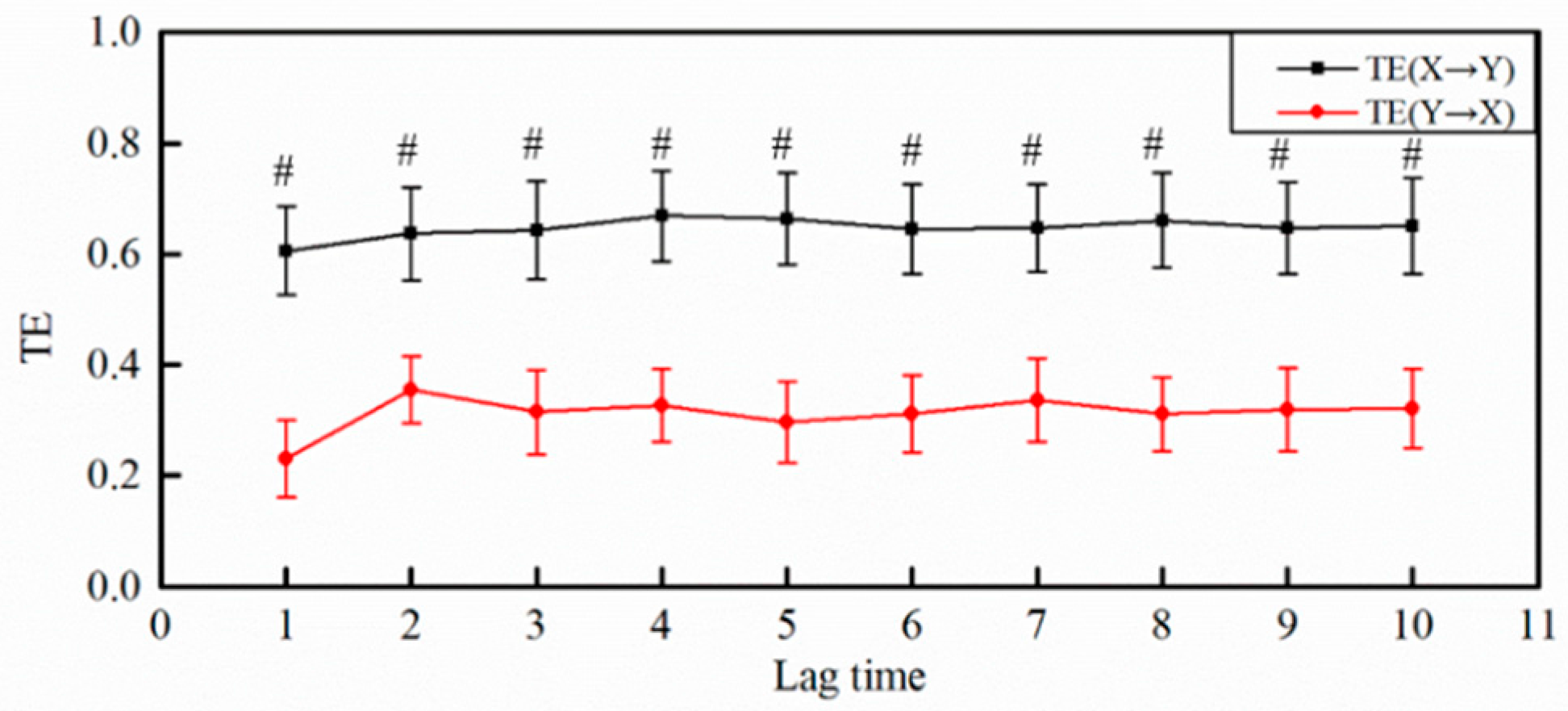
3.3.1. The Selection of Lag Time
3.3.2. The Results of MI and TE
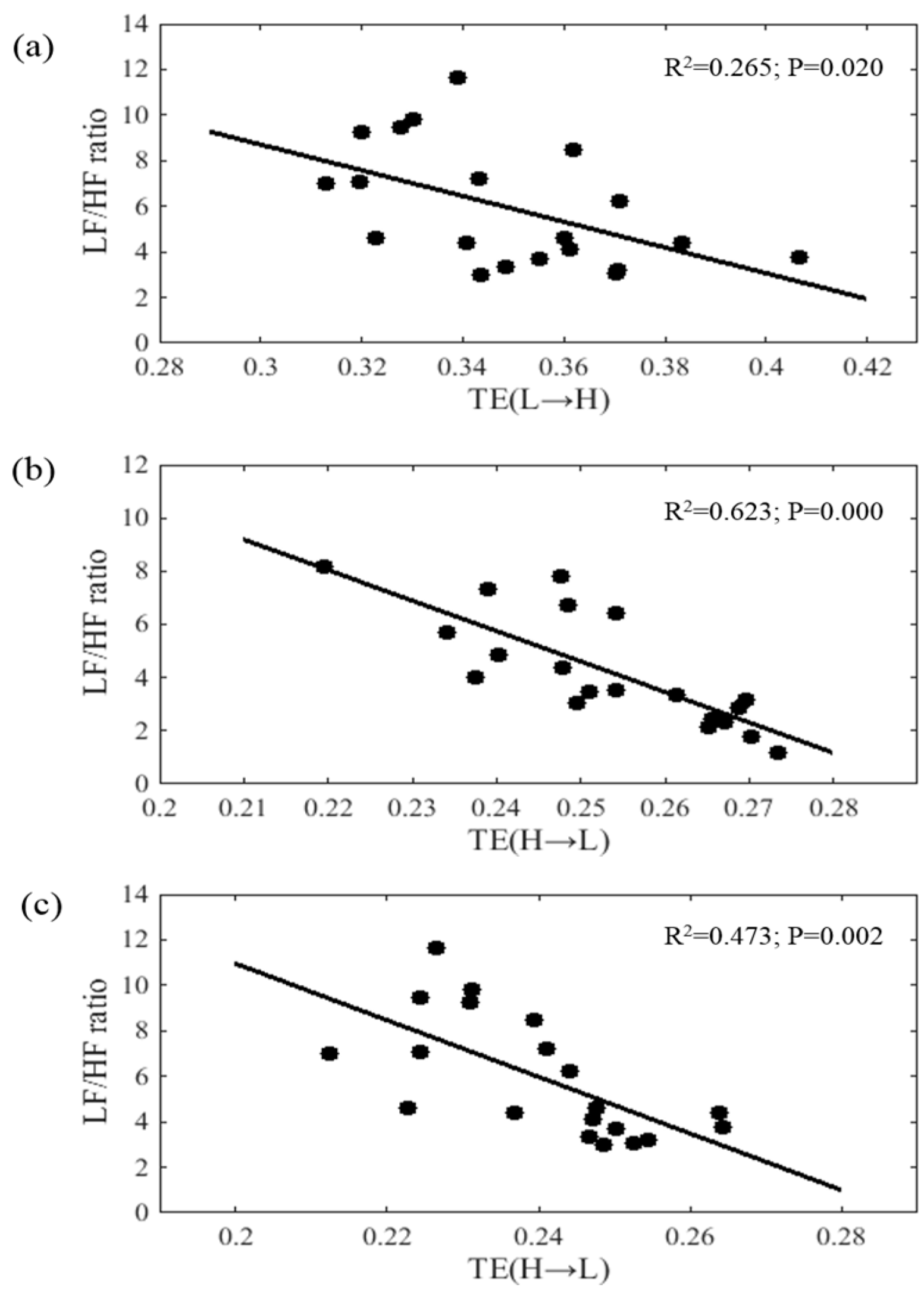
3.4 The Correlation Analysis between Interaction Features and LF/HF Ratio for Three Groups
4. Discussion
4.1. Comparison with Other Work
4.2. Analysis of Interaction Features
4.3. The Length of RRI Time Series
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, L.; Zhang, X.; Wang, H. An obstructive sleep apnea detection approach using kernel density classification based on single-lead electrocardiogram. J. Med. Syst. 2015, 39, 47. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; Bortolotto, L.A.; Lorenzi, M.C.; Figueiredo, A.C.; Krieger, E.M.; Lorenzi-Filho, G. Early signs of atherosclerosis in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2005, 172, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.S.; Peters, J.I. Congestive heart failure: Diagnosis, pathophysiology, therapy, and implications for respiratory care. Respir. Care 2006, 51, 403–412. [Google Scholar] [PubMed]
- Marshall, N.S.; Wong, K.K.; Liu, P.Y.; Cullen, S.R.; Knuiman, M.W.; Grunstein, R.R. Sleep Apnea as an Independent Risk Factor for All-Cause Mortality: The Busselton Health Study. Sleep 2008, 31, 1079–1085. [Google Scholar] [PubMed]
- Pan, W.-Y.; Su, M.-C.; Wu, H.-T.; Lin, M.-C.; Tsai, I.; Sun, C.-K. Multiscale entropy analysis of heart rate variability for assessing the severity of sleep disordered breathing. Entropy 2015, 17, 231–243. [Google Scholar] [CrossRef]
- Camm, A.J.; Malik, M.; Bigger, J.; Breithardt, G.; Cerutti, S.; Cohen, R.; Coumel, P.; Fallen, E.; Kennedy, H.; Kleiger, R. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Chen, W.; Zheng, L.; Li, K.; Qian, W.; Liu, G.; Jiang, Q. A Novel and Effective Method for Congestive Heart Failure Detection and Quantification Using Dynamic Heart Rate Variability Measurement. PLoS ONE 2016, 11, e0165304. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, Q.; Chen, S.; Zhou, G.; Chen, W.; Wu, Y. Robustness evaluation of heart rate variability measures for age gender related autonomic changes in healthy volunteers. Australas. Phys. Eng. Sci. Med. 2014, 37, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Gula, L.J.; Krahn, A.D.; Skanes, A.; Ferguson, K.A.; George, C.; Yee, R.; Klein, G.J. Heart rate variability in obstructive sleep apnea: A prospective study and frequency domain analysis. Ann. Noninvasive Electrocardiol. 2003, 8, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Lado, M.J.; Mendez, A.J.; Rodríguez-Liñares, L.; Otero, A.; Vila, X.A. Nocturnal evolution of heart rate variability indices in sleep apnea. Comput. Biol. Med. 2012, 42, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gao, R. Multiscale entropy analysis of the differential RR interval time series signal and its application in detecting congestive heart failure. Entropy 2017, 19, 251. [Google Scholar]
- Al-Angari, H.M.; Sahakian, A.V. Use of sample entropy approach to study heart rate variability in obstructive sleep apnea syndrome. IEEE Trans. Biomed. Eng. 2007, 54, 1900–1904. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.A.; Urrestarazu, E.; Lopez-Azcarate, J.; Alegre, M.; Fernandez, S.; Artieda, J.; Iriarte, J. Increased sympathetic and decreased parasympathetic cardiac tone in patients with sleep related alveolar hypoventilation. Sleep 2013, 36, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Floras, J.S.; Ponikowski, P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur. Heart J. 2015, 36, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Gammoudi, N.; Ben, C.R.; Saafi, M.A.; Sakly, G.; Dogui, M. Cardiac autonomic control in the obstructive sleep apnea. Libyan J. Med. 2015, 10, 26989. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Huang, L.; Liu, X.; Li, C.; Mao, X.; Liu, W.; Huang, X.; Chu, H.; Wang, Y.; Wu, W. Correlation Analysis between Polysomnography Diagnostic Indices and Heart Rate Variability Parameters among Patients with Obstructive Sleep Apnea Hypopnea Syndrome. PLoS ONE 2016, 11, e0156628. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-H.; Shin, C.-J.; Hong, S.-C.; Yu, J.; Ryu, S.-H.; Kim, E.-J.; Shin, H.-B.; Shin, B.-H. Correlation between the severity of obstructive sleep apnea and heart rate variability indices. J. Korean Med. Sci. 2008, 23, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Sandrone, G.; Pernpruner, S.; Sala, R.; Garimoldi, M.; Cerutti, S.; Baselli, G.; Pagani, M.; Malliani, A. Heart rate variability as an index of sympathovagal interaction after acute myocardial infarction. Am. J. Cardiol. 1987, 60, 1239–1245. [Google Scholar] [CrossRef]
- Houle, M.S.; Billman, G.E. Low-frequency component of the heart rate variability spectrum: A poor marker of sympathetic activity. Am. J. Physiol. Heart Circ. Physiol. 1999, 276, H215–H223. [Google Scholar]
- Miyamoto, T.; Kawada, T.; Yanagiya, Y.; Inagaki, M.; Takaki, H.; Sugimachi, M.; Sunagawa, K. Cardiac sympathetic nerve stimulation does not attenuate dynamic vagal control of heart rate via α-adrenergic mechanism. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H860–H865. [Google Scholar] [CrossRef] [PubMed]
- Melia, U.; Guaita, M.; Vallverdú, M.; Embid, C.; Vilaseca, I.; Salamero, M.; Santamaria, J. Mutual information measures applied to EEG signals for sleepiness characterization. Med. Eng. Phys. 2015, 37, 297. [Google Scholar] [CrossRef] [PubMed]
- Montalto, A.; Faes, L.; Marinazzo, D. MuTE: A MATLAB Toolbox to Compare Established and Novel Estimators of the Multivariate Transfer Entropy. PLoS ONE 2014, 9, e109462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Angari, H.M.; Kanitz, G.; Tarantino, S.; Cipriani, C. Distance and mutual information methods for EMG feature and channel subset selection for classification of hand movements. Biomed. Signal Process. Control 2016, 27, 24–31. [Google Scholar] [CrossRef]
- Lee, J.; Nemati, S.; Silva, I.; Edwards, B.A.; Butler, J.P.; Malhotra, A. Transfer entropy estimation and directional coupling change detection in biomedical time series. Biomed. Eng. Online 2012, 11, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faes, L.; Marinazzo, D.; Montalto, A.; Nollo, G. Lag-Specific Transfer Entropy as a Tool to Assess Cardiovascular and Cardiorespiratory Information Transfer. IEEE Trans. Biomed. Eng. 2014, 61, 2556–2568. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tompkins, W.J. A real-time QRS detection algorithm. IEEE Trans. Biomed. Eng. 1985, BME-32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Lippman, N.; Stein, K.M.; Lerman, B.B. Comparison of methods for removal of ectopy in measurement of heart rate variability. Am. J. Phys. 1994, 267, H411–H418. [Google Scholar]
- Voss, A.; Boettger, M.K.; Schulz, S.; Gross, K.; Bär, K.J. Gender-dependent impact of major depression on autonomic cardiovascular modulation. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2011, 35, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Faes, L.; Nollo, G.; Porta, A. Compensated Transfer Entropy as a Tool for Reliably Estimating Information Transfer in Physiological Time Series. Entropy 2013, 15, 198–219. [Google Scholar] [CrossRef]
- Palma, J.-A.; Iriarte, J.; Fernandez, S.; Valencia, M.; Alegre, M.; Artieda, J.; Urrestarazu, E. Characterizing the phenotypes of obstructive sleep apnea: Clinical, sleep, and autonomic features of obstructive sleep apnea with and without hypoxia. Clin. Neurophysiol. 2014, 125, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Z.; Huang, B.Y.; Wang, L. A wearable respiratory biofeedback system based on generalized body sensor network. Telemed. J. e-Health 2011, 17, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Cygankiewicz, I.; Zareba, W. Heart rate variability. Handb. Clin. Neurol. 2013, 117, 379–393. [Google Scholar] [PubMed]
- Gómez-Verdejo, V.; Verleysen, M.; Fleury, J. Information-theoretic feature selection for functional data classification. Neurocomputing 2009, 72, 3580–3589. [Google Scholar] [CrossRef]
- Trimer, R.; Mendes, R.; Costa, M.; Sampaio, M.; Delfino, A.; Arena, R.; Aletti, F.; Ferrario, M.; Borghi-Silva, A. Is there a chronic sleep stage-dependent linear and nonlinear cardiac autonomic impairment in obstructive sleep apnea? Sleep Breath. 2014, 18, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Vigo, D.E.; Guinjoan, S.M.; Scaramal, M.; Siri, L.N.; Cardinali, D.P. Wavelet transform shows age-related changes of heart rate variability within independent frequency components. Auton. Neurosci. 2005, 123, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Vigo, D.E.; Dominguez, J.; Guinjoan, S.M.; Scaramal, M.; Ruffa, E.; Solernó, J.; Siri, L.N.; Cardinali, D.P. Nonlinear analysis of heart rate variability within independent frequency components during the sleep–wake cycle. Auton. Neurosci. 2010, 154, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Jan, K.-M.; Ju, K.H.; Chon, K.H. Quantifying cardiac sympathetic and parasympathetic nervous activities using principal dynamic modes analysis of heart rate variability. Am. J. Phys. Heart Circ. Phys. 2006, 291, H1475–H1483. [Google Scholar] [CrossRef] [PubMed]
- Aktaruzzaman, M.; Sassi, R. Parametric estimation of sample entropy in heart rate variability analysis. Biomed. Signal Process. Control 2014, 14, 141–147. [Google Scholar] [CrossRef]
- Manis, G.; Aktaruzzaman, M.; Sassi, R. Bubble Entropy: An Entropy Almost Free of Parameters. IEEE Trans. Biomed. Eng. 2017. [Google Scholar] [CrossRef] [PubMed]
- De Chazal, P.; Penzel, T.; Heneghan, C. Automated detection of obstructive sleep apnoea at different time scales using the electrocardiogram. Phys. Meas. 2004, 25, 967–983. [Google Scholar] [CrossRef]
- Sharma, H.; Sharma, K.K. An algorithm for sleep apnea detection from single-lead ECG using Hermite basis functions. Comput. Biol. Med. 2016, 77, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Babaeizadeh, S.; White, D.P.; Pittman, S.D.; Zhou, S.H. Automatic detection and quantification of sleep apnea using heart rate variability. J. Electrocardiol. 2010, 43, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Varon, C.; Caicedo, A.; Testelmans, D.; Buyse, B.; Huffel, S.V. A novel algorithm for the automatic detection of sleep apnea from single-lead ECG. IEEE Trans. Biomed. Eng. 2015, 62, 2269–2278. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, K.; Zhang, X.; Chen, L.; Xian, X. An Obstructive Sleep Apnea Detection Approach Using a Discriminative Hidden Markov Model From ECG Signals. IEEE Trans. Biomed. Eng. 2016, 63, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Hyun, H.J.; Seung-Ho, R.; Yu, J.; Doo-Heum, P. The Effect of Aging and Severity of Sleep Apnea on Heart Rate Variability Indices in Obstructive Sleep Apnea Syndrome. Psychiatry Investig. 2012, 9, 65–72. [Google Scholar] [CrossRef] [PubMed]

| Parameters | LF/HF Ratio (N-N Group) | LF/HF Ratio (P-N Group) | LF/HF Ratio (P-OSA Group) | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| MI | −0.388 | 0.268 | −0.248 | 0.293 | −0.154 | 0.516 |
| TE (L→H) | −0.388 | 0.640 | −0.248 | 0.095 | −0.154 | 0.020 * |
| TE (H→L) | 0.532 | 0.113 | −0.789 | 0.000 *** | −0.661 | 0.002 ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Pan, W.; Li, Y.; Luo, D.; Wang, Q.; Liu, G. Use of Mutual Information and Transfer Entropy to Assess Interaction between Parasympathetic and Sympathetic Activities of Nervous System from HRV. Entropy 2017, 19, 489. https://doi.org/10.3390/e19090489
Zheng L, Pan W, Li Y, Luo D, Wang Q, Liu G. Use of Mutual Information and Transfer Entropy to Assess Interaction between Parasympathetic and Sympathetic Activities of Nervous System from HRV. Entropy. 2017; 19(9):489. https://doi.org/10.3390/e19090489
Chicago/Turabian StyleZheng, Lianrong, Weifeng Pan, Yifan Li, Daiyi Luo, Qian Wang, and Guanzheng Liu. 2017. "Use of Mutual Information and Transfer Entropy to Assess Interaction between Parasympathetic and Sympathetic Activities of Nervous System from HRV" Entropy 19, no. 9: 489. https://doi.org/10.3390/e19090489





