Application of Permutation Entropy and Permutation Min-Entropy in Multiple Emotional States Analysis of RRI Time Series
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Experimental Procedure and Data Collection
2.3. Permutation Entropy and Permutation Min-Entropy
2.4. Statistical Analysis
3. Results
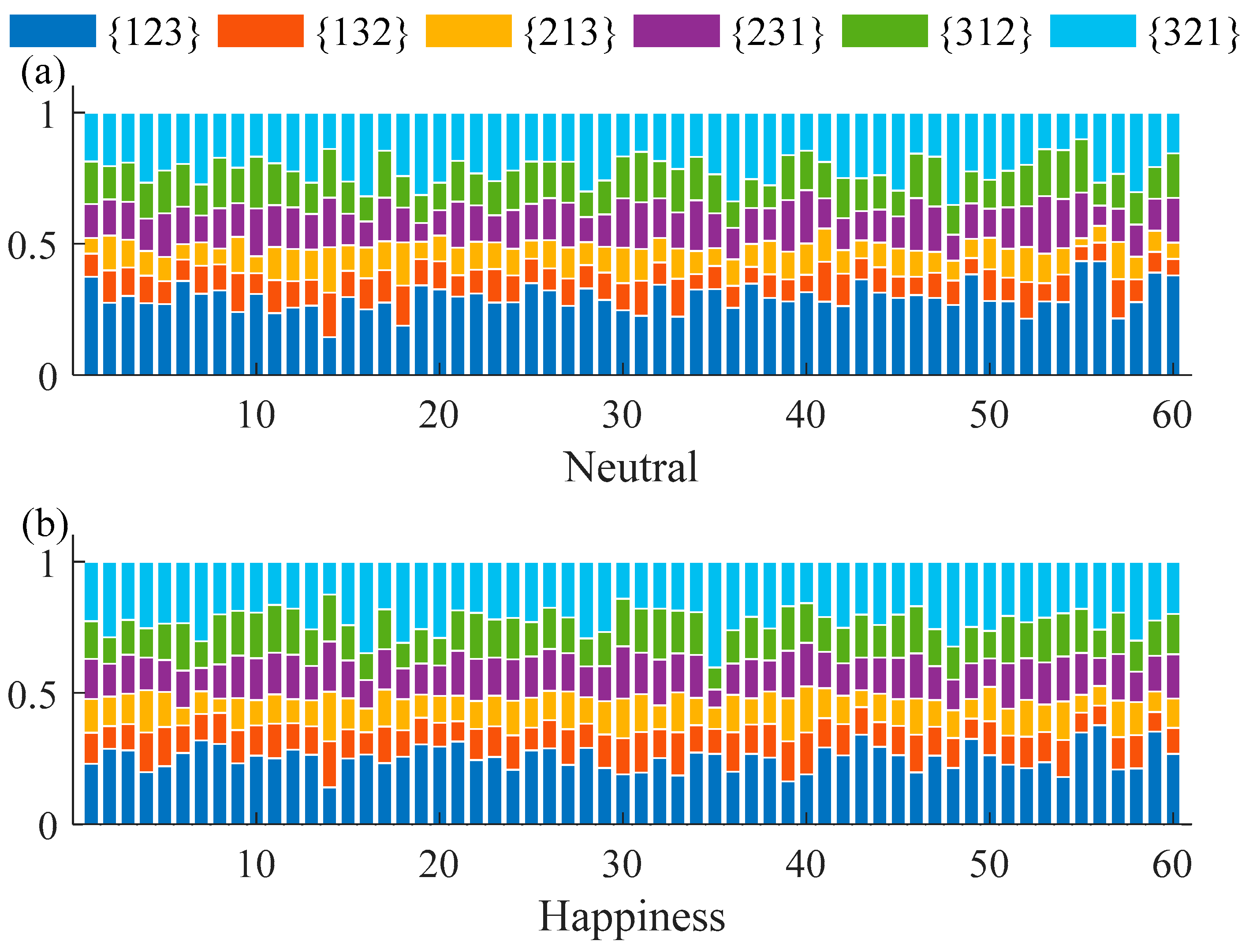
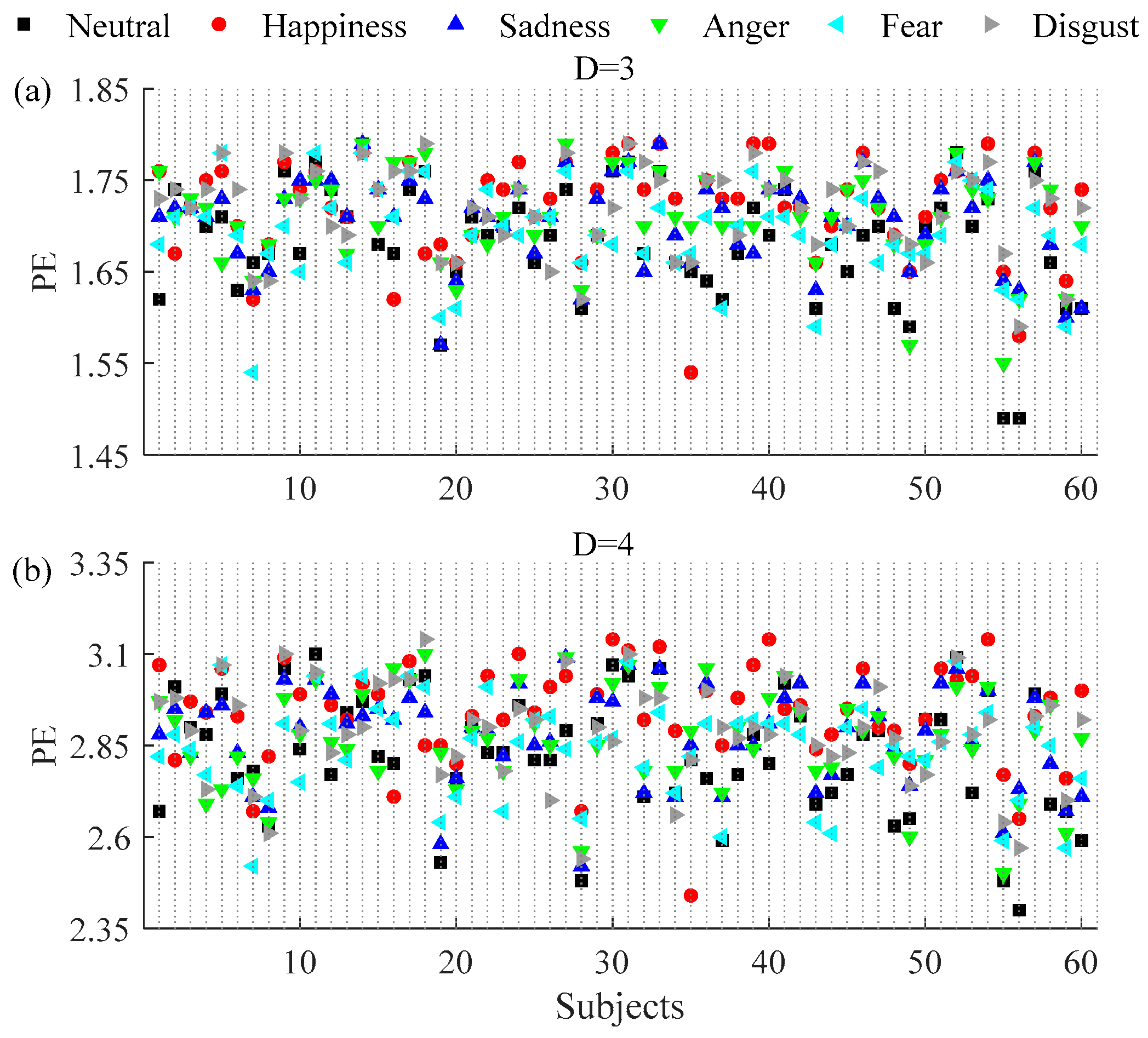
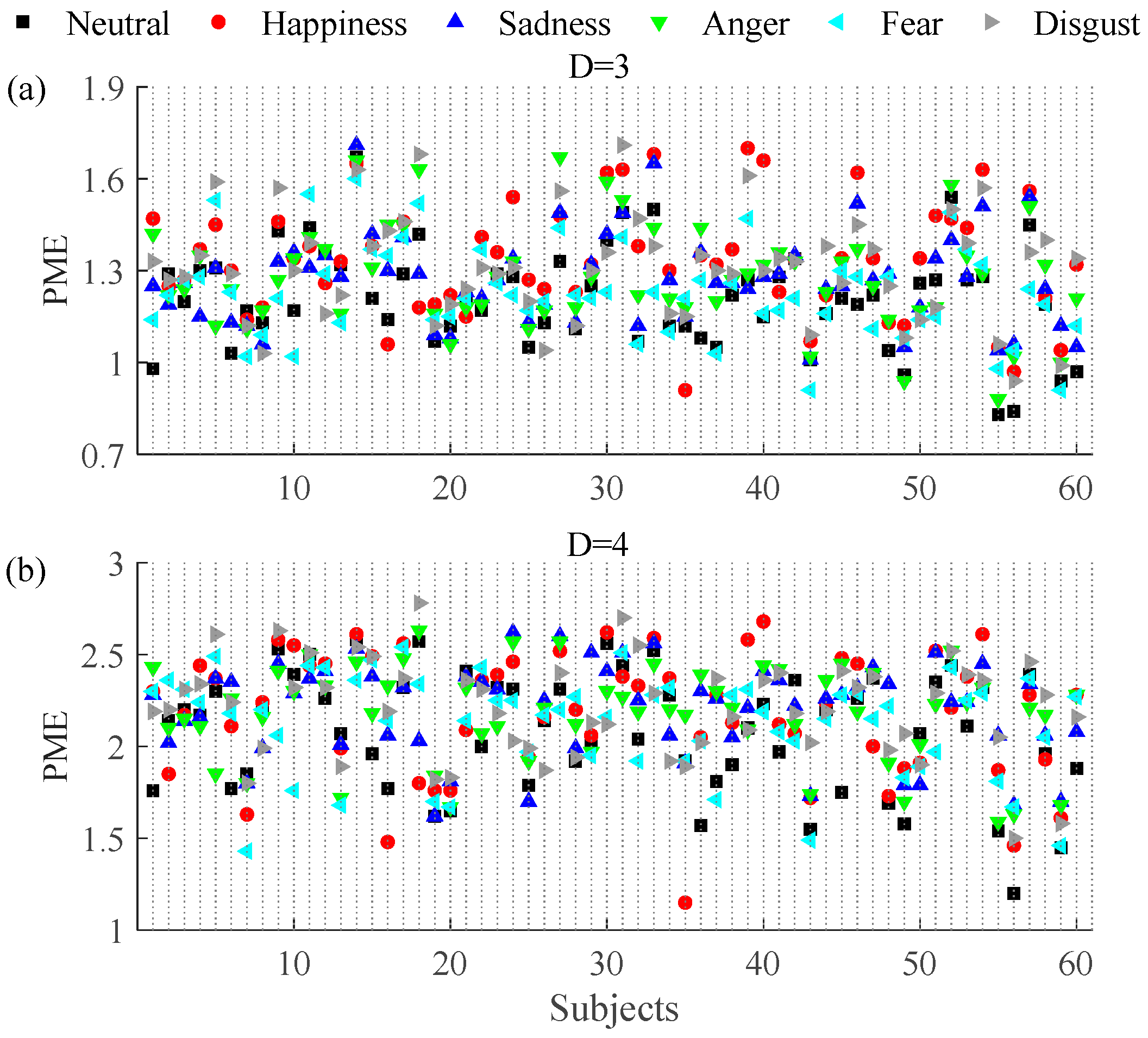
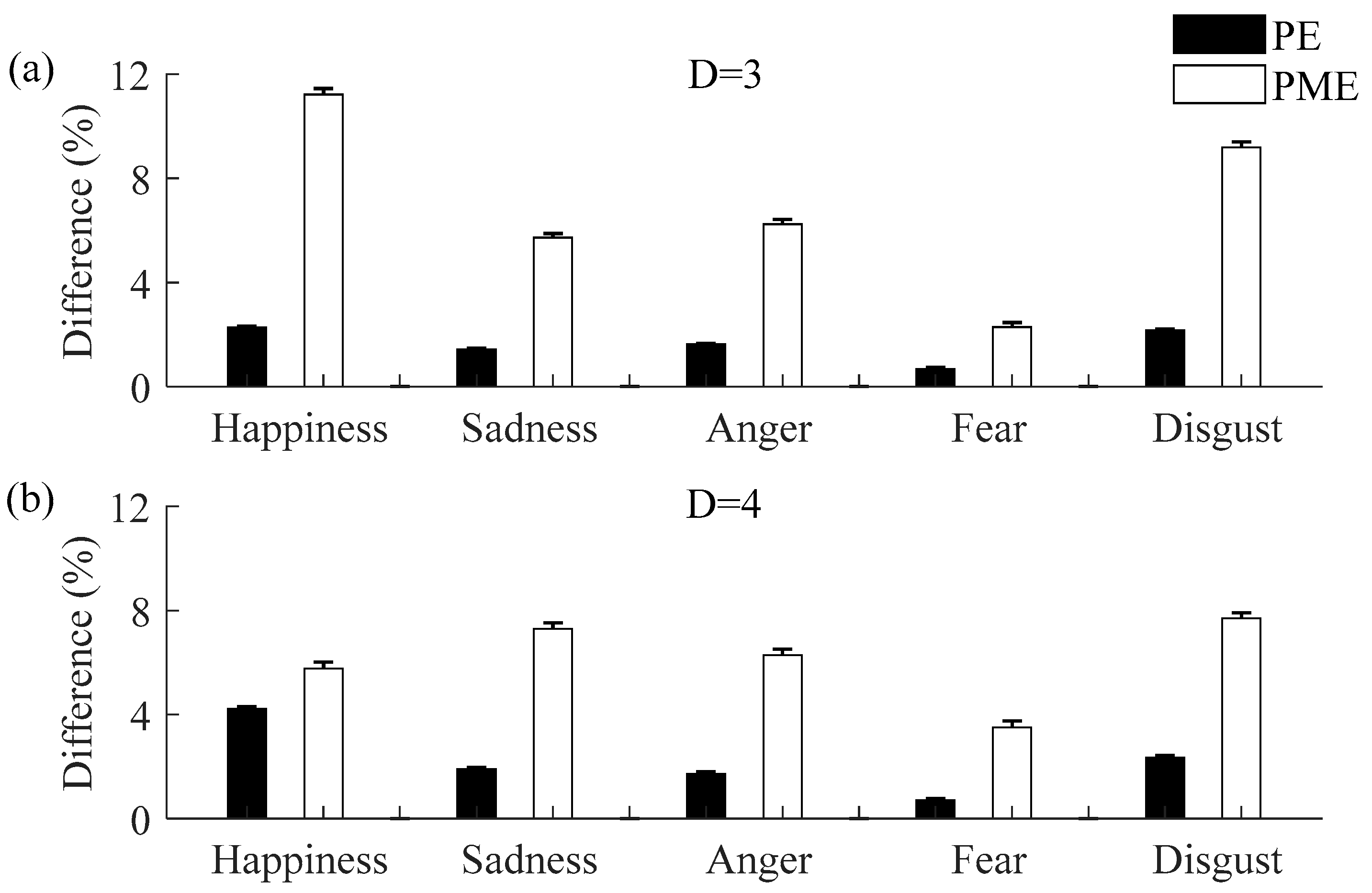
3.1. Permutation and Permutation Min-Entropy Analysis
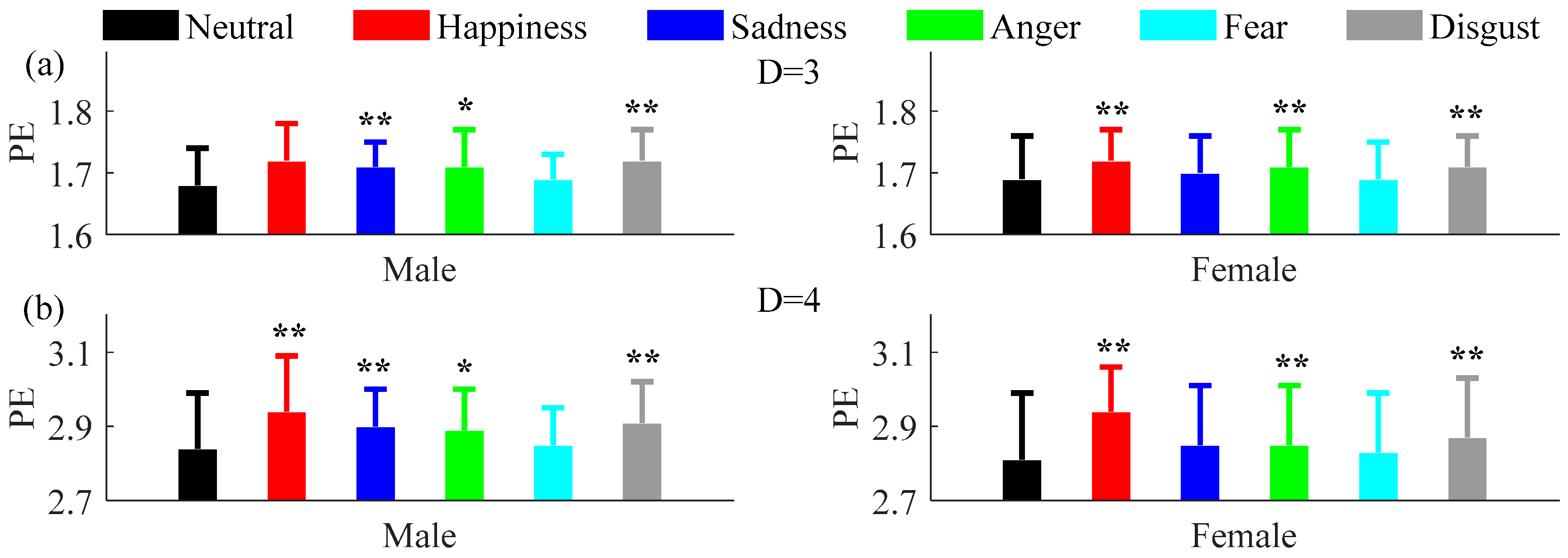
3.2. Comparisons between Neutral and Non-Neutral Emotional States
3.3. Comparisons of Permutation and Permutation Min-Entropy
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mehmood, R.M.; Lee, H.J. A novel feature extraction method based on late positive potential for emotion recognition in human brain signal patterns. Comput. Electr. Eng. 2016, 53, 444–457. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chang, C.W.; Zheng, J.Y.; Chung, P.C. Physiological emotion analysis using support vector regression. Neurocomputing 2013, 122, 79–87. [Google Scholar] [CrossRef]
- Gouizi, K.; Reguig, F.B.; Maaoui, C. Emotion recognition from physiological signals. J. Med. Eng. Technol. 2011, 35, 300–307. [Google Scholar] [CrossRef] [PubMed]
- AlZoubi, O.; Sidney, K.D.; Calvo, R.A. Detecting Naturalistic Expressions of Nonbasic Affect Using Physiological Signals. IEEE Trans. Affect. Comput. 2012, 3, 298–310. [Google Scholar] [CrossRef]
- Guo, H.W.; Huang, Y.S.; Lin, C.H.; Chien, J.C.; Haraikawa, K.; Shieh, J.S. Heart Rate Variability Signal Features for Emotion Recognition by Using Principal Component Analysis and Support Vectors Machine. In Proceedings of the 16th International Conference on Bioinformatics and Bioengineering (BIBE), Taichung, Taiwan, 31 October–2 November 2016; pp. 274–277. [Google Scholar]
- Kim, K.H.; Bang, S.W.; Kim, S.R. Emotion recognition system using short-term monitoring of physiological signals. Med. Biol. Eng. Comput. 2004, 42, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.H.; Park, B.J.; Park, M.S.; Kim, S.H.; Sohn, J.H. Analysis of physiological signals for recognition of boredom, pain, and surprise emotions. J. Physiol. Anthropol. 2015, 34, 25. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.W.; Liu, G.Y. Feature Extraction, Feature Selection and Classification from Electrocardiography to Emotions. In Proceedings of the International Conference on Computational Intelligence and Natural Computing, Wuhan, China, 6–7 June 2009; pp. 190–193. [Google Scholar]
- Valenza, G.; Lanata, A.; Scilingo, E.P. The Role of Nonlinear Dynamics in Affective Valence and Arousal Recognition. IEEE Trans. Affect. Comput. 2012, 3, 237–249. [Google Scholar] [CrossRef]
- Valenza, G.; Citi, L.; Lanata, A.; Scilingo, E.P.; Barbieri, R. Revealing Real-Time Emotional Responses: A Personalized Assessment based on Heartbeat Dynamics. Sci. Rep. 2014, 4, 4998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goshvarpour, A.; Abbasi, A.; Goshvarpour, A. Indices from lagged poincare plots of heart rate variability: An efficient nonlinear tool for emotion discrimination. Australas. Phys. Eng. Sci. Med. 2017, 40, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Bandt, C.; Pompe, B. Permutation entropy: A natural complexity measure for time series. Phys. Rev. Lett. 2002, 88, 174102. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Villecco, F.; Li, M.; Song, W. Multi-Scale Permutation Entropy Based on Improved LMD and HMM for Rolling Bearing Diagnosis. Entropy 2017, 19, 176. [Google Scholar] [CrossRef]
- Villecco, F.; Pellegrino, A. Entropic measure of epistemic uncertainties in multibody system models by axiomatic design. Entropy 2017, 19, 291. [Google Scholar] [CrossRef]
- Sena, P.; D’Amore, M.; Pappalardo, M.; Pellegrino, A.; Fiorentino, A.; Villecco, F. Studying the influence of cognitive load on driver’s performances by a Fuzzy analysis of Lane Keeping in a drive simulation. IFAC Proc. Vol. 2013, 46, 151–156. [Google Scholar] [CrossRef]
- Villecco, F.; Pellegrino, A. Evaluation of Uncertainties in the Design Process of Complex Mechanical Systems. Entropy 2017, 19, 475. [Google Scholar] [CrossRef]
- Nicolaou, N.; Georgiou, J. The Use of Permutation Entropy to Characterize Sleep Electroencephalograms. Clin. EEG Neurosci. 2011, 42, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Morabito, F.C.; Labate, D.; La Foresta, F.; Bramanti, A.; Morabito, G.; Palamara, I. Multivariate Multi-Scale Permutation Entropy for Complexity Analysis of Alzheimer’s Disease EEG. Entropy 2012, 14, 1186–1202. [Google Scholar] [CrossRef]
- Li, J.; Yan, J.; Liu, X.; Ouyang, G. Using Permutation Entropy to Measure the Changes in EEG Signals During Absence Seizures. Entropy 2014, 16, 3049–3061. [Google Scholar] [CrossRef]
- Martin, J.; Schneider, F.; Kowalewskij, A.; Jordan, D.; Hapfelmeier, A.; Kochs, E.; Wagner, K.; Schulz, C. Linear and non-linear heart rate metrics for the assessment of anaesthetists’ workload during general anaesthesia. Br. J. Anaesth. 2016, 117, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Aziz, W.; Arif, M. Multiscale permutation entropy of physiological time series. In Proceedings of the 9th International Conference of Multitopic, Karachi, Pakistan, 24–25 December 2005; pp. 368–373. [Google Scholar]
- Graff, B.; Graff, G.; Makowiec, D.; Kaczkowska, A.; Wejer, D.; Budrejko, S.; Kozłowski, D.; Narkiewicz, K. Entropy measures in the assessment of heart rate variability in patients with cardiodepressive vasovagal syncope. Entropy 2015, 17, 1007–1022. [Google Scholar] [CrossRef]
- García-Martínez, B.; Martínez-Rodrigo, A.; Zangróniz, R.; Pastor, J.M.; Alcaraz, R. Symbolic analysis of brain dynamics detects negative stress. Entropy 2017, 19, 196. [Google Scholar] [CrossRef]
- Zunino, L.; Olivares, F.; Rosso, O.A. Permutation min-entropy: An improved quantifier for unveiling subtle temporal correlations. EPL 2015, 109, 10005. [Google Scholar] [CrossRef]
- Rioul, O.; Martin, V. Wavelets and signal processing. IEEE Signal Process. Mag. 1991, 8, 14–38. [Google Scholar] [CrossRef]
- Pan, J.; Tompkins, W.J. A real-time QRS detection algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Zunino, L.; Olivares, F.; Scholkmann, F.; Rosso, O.A. Permutation entropy based time series analysis: Equalities in the input signal can lead to false conclusions. Phys. Lett. 2017, 381, 1883–1892. [Google Scholar] [CrossRef]
- Graff, G.; Graff, B.; Kaczkowska, A.; Makowiec, D.; Amigó, J.; Piskorski, J.; Narkiewicz, K.; Guzik, P. Ordinal pattern statistics for the assessment of heart rate variability. Eur. Phys. J. Spec. Top. 2013, 222, 525–534. [Google Scholar] [CrossRef]
- Chendi, W.; Feng, W. An emotional analysis method based on heart rate variability. In Proceedings of the 2012 IEEE-EMBS International Conference on Biomedical and Health Informatics, Hong Kong, China, 5–7 January 2012; pp. 104–107. [Google Scholar]
- Valenza, G.; Allegrini, P.; Lanata, A.; Scilingo, E.P. Dominant Lyapunov exponent and approximate entropy in heart rate variability during emotional visual elicitation. Front. Neuroeng. 2012, 5, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalano, J.T. Guide to ECG; J.B. Lippincott: Philadelphia, PA, USA, 1993. [Google Scholar]
- Porta, A.; Gnecchi-Ruscone, T.; Tobaldini, E.; Guzzetti, S.; Furlan, R.; Montano, N. Progressive decrease of heart period variability entropy-based complexity during graded head-up tilt. J. Appl. Physiol. 2007, 103, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Porta, A.; Castiglioni, P.; Bari, V.; Bassani, T.; Marchi, A.; Cividjian, A.; Quintin, L.; Di Rienzo, M. K-nearest-neighbor conditional entropy approach for the assessment of the short-term complexity of cardiovascular control. Physiol. Meas. 2013, 34, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Weippert, M.; Behrens, M.; Rieger, A.; Behrens, K. Sample Entropy and Traditional Measures of Heart Rate Dynamics Reveal Different Modes of Cardiovascular Control During Low Intensity Exercise. Entropy 2014, 16, 5698–5711. [Google Scholar] [CrossRef]




| Variables | Male | Female | Total |
|---|---|---|---|
| Number of subjects | 30 | 30 | 60 |
| Age (year) | 23 ± 1 | 23 ± 2 | 23 ± 2 |
| Height (cm) | 175 ± 5 | 163 ± 4 | 169 ± 8 |
| Weight (kg) | 69 ± 8 | 53 ± 6 | 61 ± 11 |
| Body mass index (kg/m2) | 22 ± 3 | 20 ± 2 | 21 ± 3 |
| Heart rate (beats/min) | 77 ± 11 | 78 ± 11 | 78 ± 11 |
| Systolic blood pressure (mmHg) | 123 ± 10 | 107 ± 7 | 116 ± 12 |
| Diastolic blood pressure (mmHg) | 76 ± 8 | 70 ± 6 | 73 ± 8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Yang, L.; Zunino, L.; Shi, H.; Zhuang, Y.; Liu, C. Application of Permutation Entropy and Permutation Min-Entropy in Multiple Emotional States Analysis of RRI Time Series. Entropy 2018, 20, 148. https://doi.org/10.3390/e20030148
Xia Y, Yang L, Zunino L, Shi H, Zhuang Y, Liu C. Application of Permutation Entropy and Permutation Min-Entropy in Multiple Emotional States Analysis of RRI Time Series. Entropy. 2018; 20(3):148. https://doi.org/10.3390/e20030148
Chicago/Turabian StyleXia, Yirong, Licai Yang, Luciano Zunino, Hongyu Shi, Yuan Zhuang, and Chengyu Liu. 2018. "Application of Permutation Entropy and Permutation Min-Entropy in Multiple Emotional States Analysis of RRI Time Series" Entropy 20, no. 3: 148. https://doi.org/10.3390/e20030148







