The Effects of Mo and Nb on the Microstructures and Properties of CrFeCoNi(Nb,Mo) Alloys
Abstract
:1. Introduction
2. Experimental
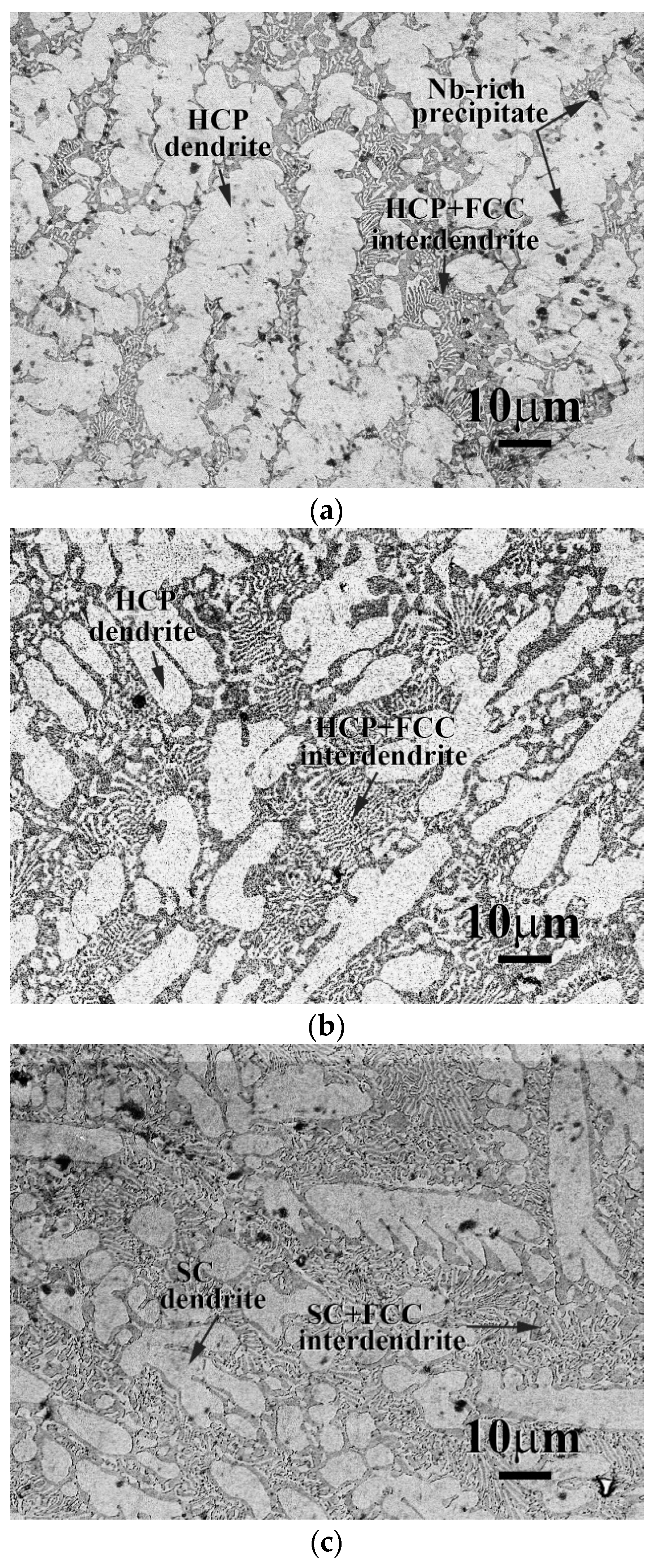
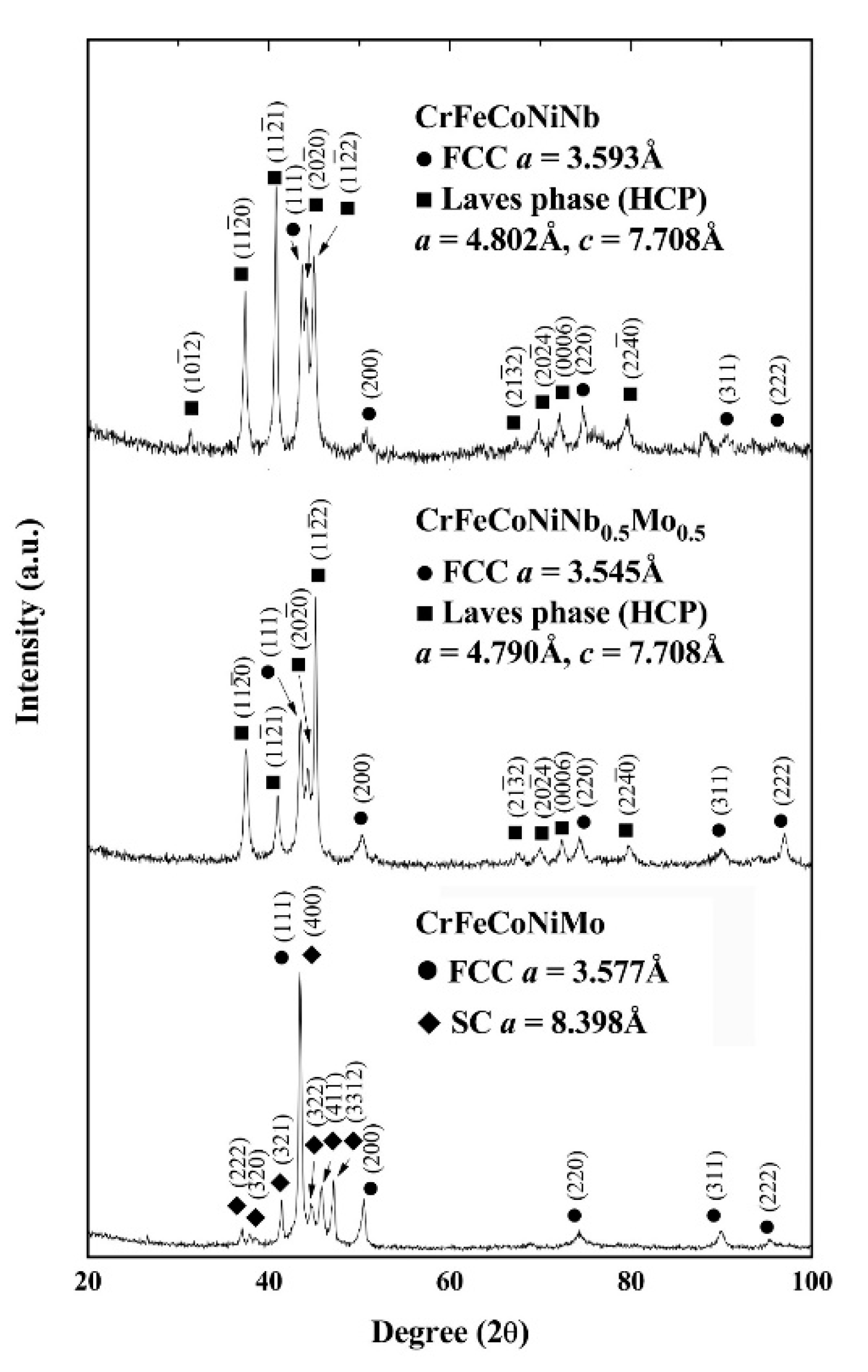
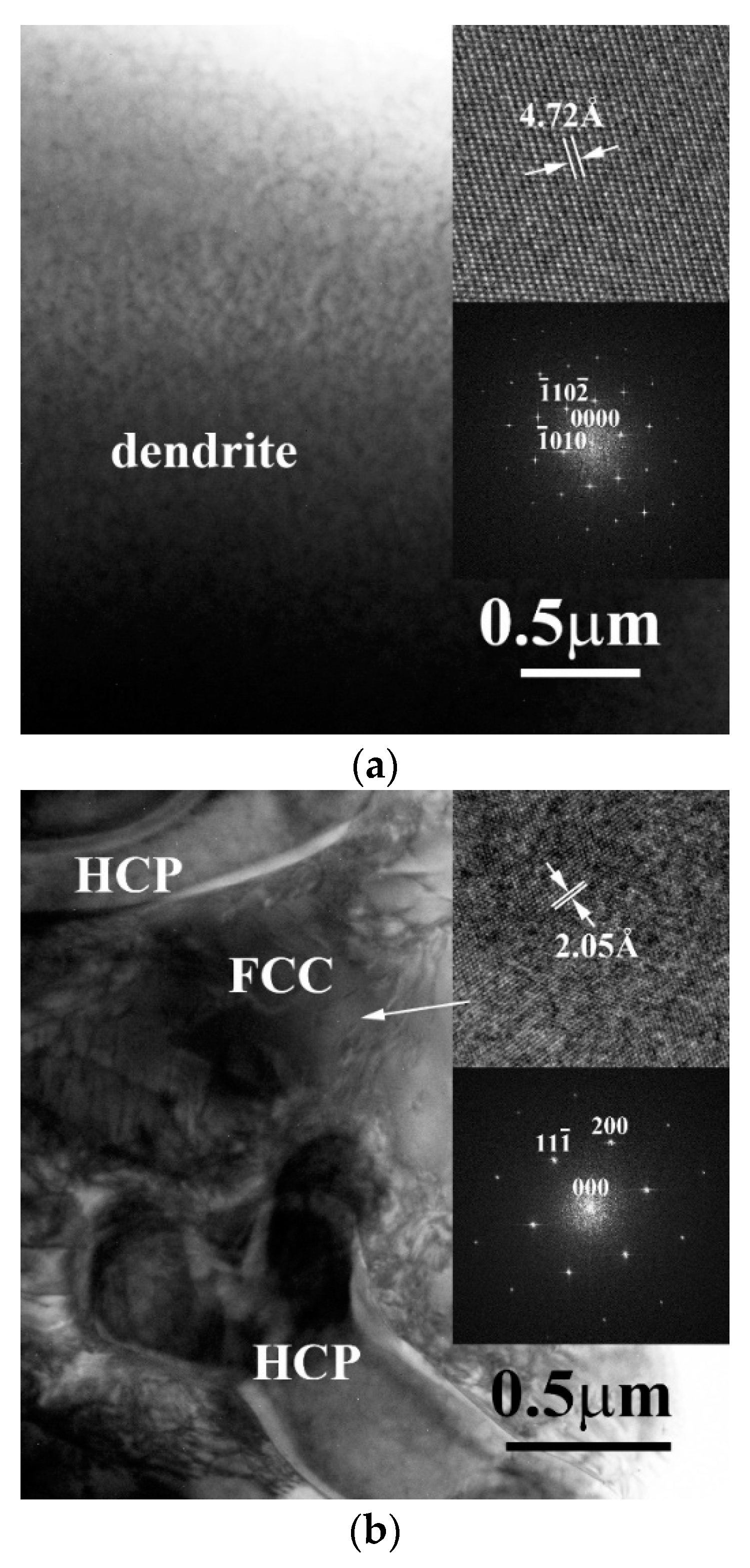
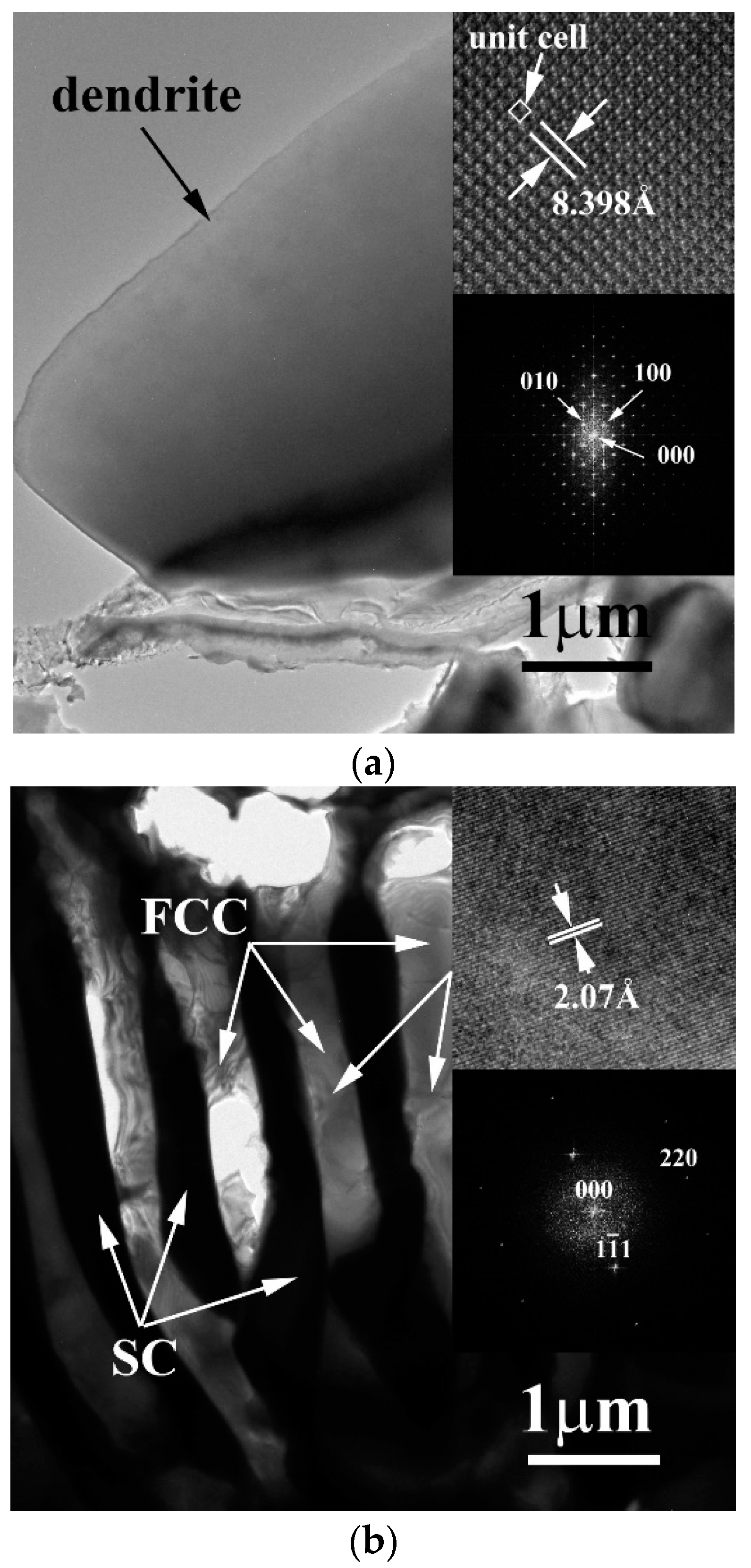
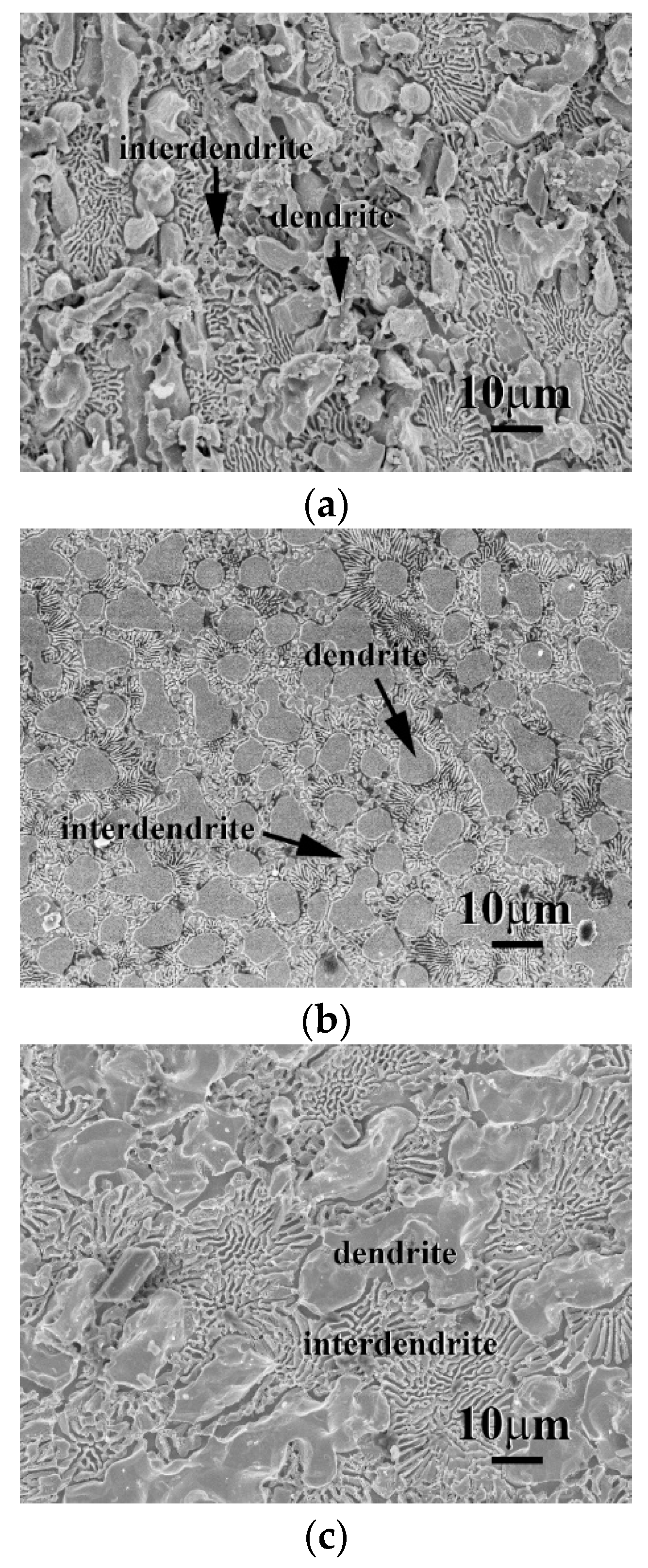
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S. High-Entropy Alloys; Butterworth-Heinemann: London, UK, 2014; pp. 13–36. [Google Scholar]
- Zhang, W.; Liaw, P.K.; Zhang, Y. Science and technology in high-entropy alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.M.; Lin, S.J.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Chen, H.C. Adhesive wear behavior of AlxCoCrCuFeNi high-entropy alloys as a function of aluminum content. Wear 2006, 261, 513–519. [Google Scholar] [CrossRef]
- Huo, W.Y.; Shi, H.F.; Ren, X.; Zhang, J.Y. Microstructure and wear behavior of CoCrFeMnNbNi High-Entropy Alloy Coating by TIG Cladding. Adv. Mater. Sci. Eng. 2015, 2015, 647351. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Gao, M.C.; Carney, C.S.; Doğan, Ő.N.; Jablonksi, P.D.; Hawk, J.A.; Alman, D.E. Design of refractory high-entropy alloys. JOM 2015, 67, 2653–2669. [Google Scholar] [CrossRef]
- Antonaglia, J.; Xie, X.; Tang, Z.; Tsai, C.W.; Qiao, J.W.; Zhang, Y.; Laktionova, M.O.; Tabachnikova, E.D.; Yeh, J.W.; Senkov, O.N.; et al. Temperature effects on deformation and serration behavior of high-entropy alloys (HEAs). JOM 2014, 66, 2002–2008. [Google Scholar]
- Sheng, W.J.; Yang, X.; Wang, C.; Zhang, Y. Nano-Crystallization of high-entropy amorphous NbTiAlSiWxNy films prepared by magnetron sputtering. Entropy 2016, 18, 226. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, X.-H.; Liao, W.-B.; Zhao, K. Effects of nitrogen content on the structure and mechanical properties of (Al0.5CrFeNiTi0.25)Nx high-entropy films by reactive sputtering. Entropy 2018, 20, 624. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Chiang, W.C.; Wu, J.K. Corrosion behavior of FeCoNiCrCux high-entropy alloys in 3.5% sodium chloride solution. Mater. Chem. Phys. 2005, 92, 112–117. [Google Scholar] [CrossRef]
- Tsau, C.H.; Lee, P.Y. Microstructures of Al7.5Cr22.5Fe35Mn20Ni15 high-entropy alloy and its polarization behaviors in sulfuric acid, nitric acid and hydrochloric acid solutions. Entropy 2016, 18, 288. [Google Scholar] [CrossRef]
- Lin, C.M.; Tsai, H.L. Evolution of microstructure, hardness, and corrosion properties of high-entropy Al0.5CoCrFeNi alloy. Intermetallics 2011, 19, 288–294. [Google Scholar] [CrossRef]
- Tsau, C.H.; Lin, S.X.; Fang, C.H. Microstructures and corrosion behaviors of FeCoNi and CrFeCoNi equimolar alloys. Mater. Chem. Phys. 2017, 186, 534–540. [Google Scholar] [CrossRef]
- Hashimoto, K.; Asami, K.; Teramoto, K. An X-ray photo-electron spectroscopic study on the role of molybdenum in increasing the corrosion resistance of ferritic stainless steels in HCl. Corros. Sci. 1979, 19, 3–14. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Viejo, F.; Arrabal, R.; Matykina, E. Effect of Mo and Mn additions on the corrosion behavior of AISI 304 and 316 stainless steel in H2SO4. Cossos. Sci. 2008, 50, 780–794. [Google Scholar]
- Mariano, N.A.; Souza, C.A.C.; May, J.E.; Kuri, S.E. Influence of Nb content on the corrosion resistance and saturation magnetic density of FeCuNbSiB alloys. Mater. Sci. Eng. A 2003, 354, 1–5. [Google Scholar] [CrossRef]
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 31–63. [Google Scholar]
- Voort, G.F.V. Metallography-Principles and Practice; ASM International: Materials Park, OH, USA, 1999; pp. 425–426. [Google Scholar]
- Smith, W.F. Foundations of Materials Science and Engineering, 3rd ed; McGraw-Hill: New York, NY, USA, 2004; pp. 877–878. [Google Scholar]
- Chawla, S.L. Materials Selection for Corrosion Control; ASM International: Materials Park, OH, USA, 1993; p. 18. [Google Scholar]
- Abdallah, M. Corrosion behavior of 304 stainless steel in sulphuric acid solutions and its inhibition by some substituted pyrazolones. Mater. Chem. Phys. 2003, 81, 786–792. [Google Scholar] [CrossRef]
- Tomio, A.; Sagara, M.; Doi, T.; Amaya, H.; Otsuka, N.; Kudo, T. Role of alloyed molybdenum on corrosion resistance of austenitic Ni-Cr-Mo-Fe alloys in H2S-Cl− environments. Corros. Sci. 2015; 98, 391–398. [Google Scholar]





| Alloys | Compositions (Atomic Percent) | |||||
|---|---|---|---|---|---|---|
| Cr | Fe | Co | Ni | Nb | Mo | |
| CrFeCoNiMo | 20.1 | 20.2 | 19.5 | 20.5 | N/A | 19.7 |
| CrFeCoNiNb0.5Mo0.5 | 21.4 | 19.4 | 19.8 | 17.6 | 11.0 | 10.8 |
| CrFeCoNiNb | 19.8 | 19.8 | 19.0 | 19.5 | 22.0 | N/A |
| Alloys | Compositions (Atomic Percent) | |||||
|---|---|---|---|---|---|---|
| Co | Cr | Fe | Ni | Mo | Nb | |
| CrFeCoNiNb | ||||||
| FCC | 18.4 | 26.7 | 24.3 | 25.5 | N/A | 5.1 |
| HCP | 19.7 | 17.1 | 18.1 | 14.8 | N/A | 30.3 |
| precipitate | 19.1 | 16.7 | 16.7 | 15.7 | N/A | 31.8 |
| CrFeCoNiNb0.5Mo0.5 | ||||||
| FCC | 18.4 | 22.1 | 20.4 | 22.8 | 9.2 | 7.1 |
| HCP | 19.1 | 17.3 | 23.6 | 14.2 | 15.6 | 16.9 |
| CrFeCoNiMo | ||||||
| FCC | 21.3 | 19.2 | 22.0 | 24.0 | 13.5 | N/A |
| SC | 17.2 | 21.9 | 17.7 | 14.4 | 28.9 | N/A |
| Alloys | Volume Fraction of the Dendrites (vol.%) |
|---|---|
| CrFeCoNiMo | 42 ± 6 |
| CrFeCoNiNb0.5Mo0.5 | 36 ± 6 |
| CrFeCoNiNb | 68 ± 4 |
| Alloys | Hardness | ||
|---|---|---|---|
| Overall | Dendrite | Interdendrite | |
| CrFeCoNiMo | 604 ± 8 | 692 ± 18 | 405 ± 9 |
| CrFeCoNiNb0.5Mo0.5 | 533 ± 6 | 745 ± 10 | 412 ± 7 |
| CrFeCoNiNb | 652 ± 8 | 693 ± 23 | 398 ± 24 |
| Alloys | icorr μA/cm2 | Ecorr V vs. SSE | Epp V vs. SSE | icrit mA/cm2 | ipass μA/cm2 |
|---|---|---|---|---|---|
| CrFeCoNiNb | 22.3 | −0.290 | −0.090 | 0.028 | 12.4 |
| CrFeCoNiNb0.5Mo0.5 | 12.9 | −0.256 | −0.165 | 0.022 | 12.2 |
| CrFeCoNiMo | 30.0 | −0.236 | −0.174 | 0.013 | 18.9 |
| 304SS | 30.0 | −0.320 | −0.140 | 0.930 | 17.2 |
| Reaction | Electrode Potential (E° vs. SSE) |
|---|---|
| Cr, Cr3+ | −0.962 |
| Fe, Fe2+ | −0.662 |
| Co, Co2+ | −0.449 |
| Ni, Ni2+ | −0.472 |
| Nb, Nb3+ | −1.322 |
| Mo, Mo3+ | −0.422 |
| Alloys | icorr | Ecorr |
|---|---|---|
| A/cm2 | V vs. SSE | |
| CrFeCoNiNb | 1.2 | −0.443 |
| CrFeCoNiNb0.5Mo0.5 | 6.7 | −0.477 |
| CrFeCoNiMo | 13.0 | −0.489 |
| 304SS | 12.9 | −0.860 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsau, C.-H.; Tsai, M.-C. The Effects of Mo and Nb on the Microstructures and Properties of CrFeCoNi(Nb,Mo) Alloys. Entropy 2018, 20, 648. https://doi.org/10.3390/e20090648
Tsau C-H, Tsai M-C. The Effects of Mo and Nb on the Microstructures and Properties of CrFeCoNi(Nb,Mo) Alloys. Entropy. 2018; 20(9):648. https://doi.org/10.3390/e20090648
Chicago/Turabian StyleTsau, Chun-Huei, and Meng-Chi Tsai. 2018. "The Effects of Mo and Nb on the Microstructures and Properties of CrFeCoNi(Nb,Mo) Alloys" Entropy 20, no. 9: 648. https://doi.org/10.3390/e20090648




