Complexity-Based Measures of Postural Sway during Walking at Different Speeds and Durations Using Multiscale Entropy
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Experimental Procedures
2.3. Center of Pressure Measurements
2.4. Multiscale Entropy Analysis
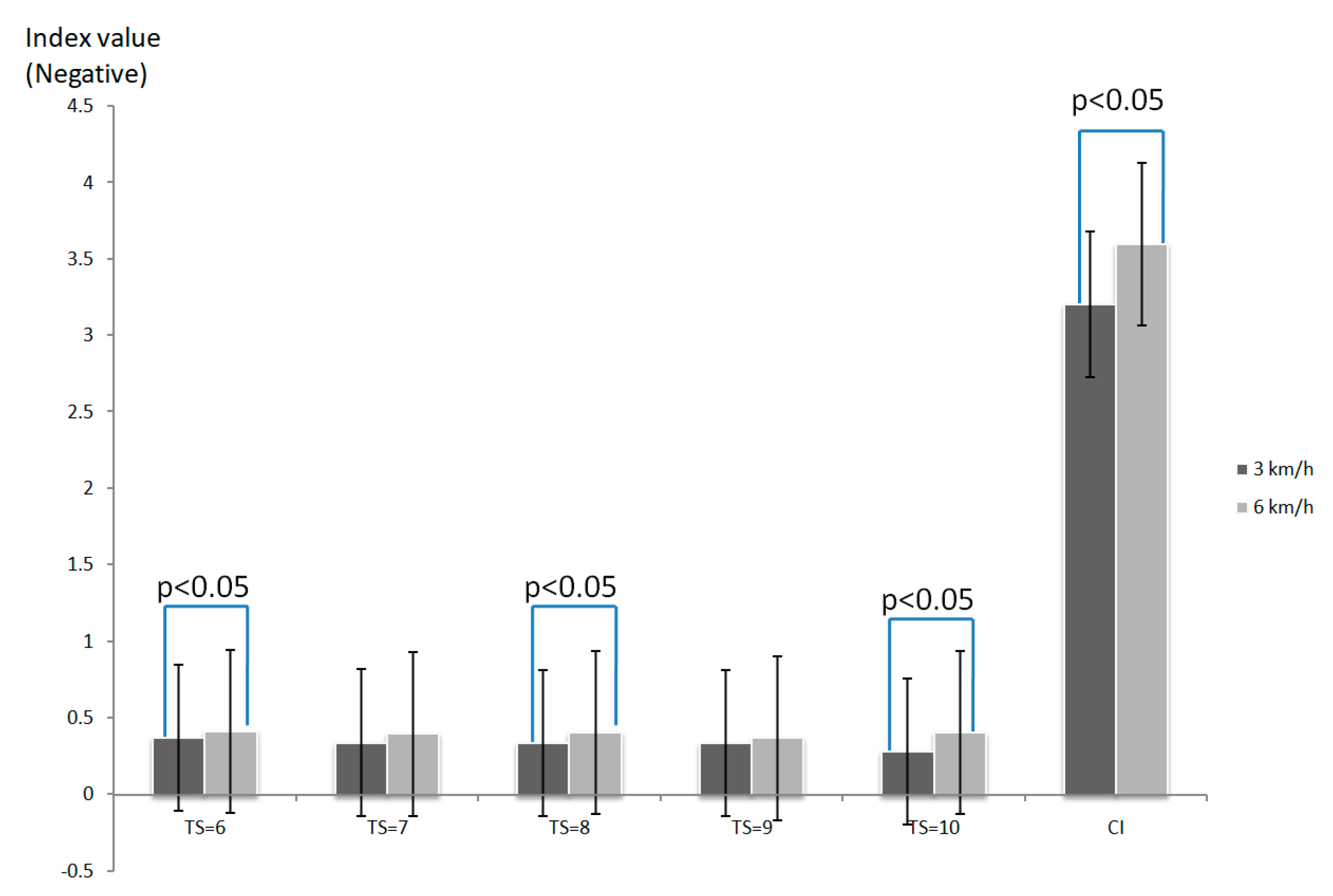
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Gow, B.J.; Peng, C.K.; Wayne, P.M.; Ahn, A.C. Multiscale Entropy Analysis of Center-of-Pressure Dynamics in Human Postural Control: Methodological Considerations. Entropy 2015, 17, 7926–7947. [Google Scholar] [CrossRef]
- Hu, X.Y.; Zhao, J.; Peng, D.S.; Sun, Z.L.; Qu, X.D. Estimation of Foot Plantar Center of Pressure Trajectories with Low-Cost Instrumented Insoles Using an Individual-Specific Nonlinear Model. Sensors 2018, 18, 421. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Sue, P.D.; Abbod, M.F.; Jiang, B.C.; Shieh, J.S. Measuring Center of Pressure Signals to Quantify Human Balance Using Multivariate Multiscale Entropy by Designing a Force Platform. Sensors 2013, 13, 10151–10166. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Liu, D.H.; Wang, K.H.; Liu, Q.; Abbod, M.F.; Jiang, B.C.; Chen, K.P.; Wu, C.; Shieh, J.S. Multivariate Multiscale Entropy Applied to Center of Pressure Signals Analysis: An Effect of Vibration Stimulation of Shoes. Entropy 2012, 14, 2157–2172. [Google Scholar] [CrossRef]
- Grant, P.M.; Dall, P.M.; Kerr, A. Daily and hourly frequency of the sit to stand movement in older adults: A comparison of day hospital, rehabilitation ward and community living groups. Aging Clin. Exp. Res. 2011, 23, 437–444. [Google Scholar] [CrossRef]
- Bestaven, E.; Petit, J.; Robert, B.; Dehail, P. Center of pressure path during Sit-to-walk tasks in young and elderly humans. Ann. Phys. Rehabi. Med. 2013, 56, 644–651. [Google Scholar] [CrossRef]
- Baratto, L.; Morasso, P.G.; Re, C.; Spada, G. A new look at posturographic analysis in the clinical context: Sway-density versus other parameterization techniques. Motor. Control. 2002, 6, 246–270. [Google Scholar] [CrossRef]
- Bauer, C.; Groger, I.; Rupprecht, R.; Gassmann, K.G. Intrasession Reliability of Force Platform Parameters in Community-Dwelling Older Adults. Arch. Phys. Med. Rehabil. 2008, 89, 1977–1982. [Google Scholar] [CrossRef]
- Corriveau, H.; Hebert, R.; Raiche, M.; Prince, F. Evaluation of postural stability in the elderly with stroke. Arch. Phys. Med. Rehabil. 2004, 85, 1095–1101. [Google Scholar] [CrossRef]
- Paillard, T.; Noe, F. Techniques and Methods for Testing the Postural Function in Healthy and Pathological Subjects. BioMed Res. Int. 2015. [Google Scholar] [CrossRef]
- Fino, P.C.; Mojdehi, A.R.; Adjerid, K.; Habibi, M.; Lockhart, T.E.; Ross, S.D. Comparing Postural Stability Entropy Analyses to Differentiate Fallers and Non-fallers. Ann. Biomed. Eng. 2016, 44, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Busa, M.A.; Jones, S.L.; Hamill, J.; van Emmerik, R.E.A. Multiscale entropy identifies differences in complexity in postural control in women with multiple sclerosis. Gait Posture 2016, 45, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.Y.; Yang, T.D.; Wu, F.L.; Cao, C.M.; Mohamed, A.; Jan, Y.K. Using Multiscale Entropy to Assess the Efficacy of Local Cooling on Reactive Hyperemia in People with a Spinal Cord Injury. Entropy 2019, 21, 90. [Google Scholar] [CrossRef]
- Liao, F.Y.; Struck, B.D.; MacRobert, M.; Jan, Y.K. Multifractal analysis of nonlinear complexity of sacral skin blood flow oscillations in older adults. Med. Biol. Eng. Comput. 2011, 49, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.Y.; Jan, Y.K. Using multifractal detrended fluctuation analysis to assess sacral skin blood flow oscillations in people with spinal cord injury. J. Rehabil. Res. Dev. 2011, 48, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Jan, Y.K.; Liao, F.Y.; Cheing, G.L.Y.; Pu, F.; Ren, W.Y.; Choi, H.M.C. Differences in skin blood flow oscillations between the plantar and dorsal foot in people with diabetes mellitus and peripheral neuropathy. Microvasc. Res. 2019, 122, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Isableu, B.; Hlavackova, P.; Diot, B.; Vuillerme, N. Regularity of Center of Pressure Trajectories in Expert Gymnasts during Bipedal Closed-Eyes Quiet Standing. Front. Hum. Neurosci. 2017, 11, 317. [Google Scholar] [CrossRef]
- Jelinek, H.F.; Khalaf, K.; Poilvet, J.; Khandoker, A.H.; Heale, L.; Donnan, L. The Effect of Ankle Support on Lower Limb Kinematics During the Y-Balance Test Using Non-linear Dynamic Measures. Front. Physiol. 2019, 10, 935. [Google Scholar] [CrossRef]
- Raffalt, P.C.; Chrysanthou, M.; Duda, G.N.; Agres, A.N. Dynamics of postural control in individuals with ankle instability: Effect of visual input and orthotic use. Comput. Biol. Med. 2019, 110, 120–126. [Google Scholar] [CrossRef]
- Mirahmadi, M.; Karimi, M.T.; Esrafilian, A. An Evaluation of the Effect of Vision on Standing Stability in the Early Stage of Parkinson’s Disease. Eur. Neurol. 2018, 80, 261–267. [Google Scholar] [CrossRef]
- Li, Y.; Mache, M.A.; Todd, T.A. Complexity of Center of Pressure in Postural Control for Children with Autism Spectrum Disorders was Partially Compromised. J. Appl. Biomech. 2019, 35, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, S.; Adair, H.; Woodruff, A.; Ryan, L.J.; Williams, B.; James, E.; Bell, K.R. Balance Testing Following Concussion: Postural Sway versus Complexity Index. PM&R 2019. [Google Scholar] [CrossRef]
- Bizovska, L.; Janura, M.; Svoboda, Z.; Cerny, M.; Krohova, J.; Smondrk, M. Intra- and inter-session reliability of traditional and entropy-based variables describing stance on a wobble board. Med. Eng. Phys. 2017, 50, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Ivanov, K.; Zhao, G.; Li, H.; Wang, L. An explorative investigation of functional differences in plantar center of pressure of four foot types using sample entropy method. Med. Biol. Eng. Comput. 2017, 55, 537–548. [Google Scholar] [CrossRef]
- Mei, Z.; Zhao, G.; Ivanov, K.; Guo, Y.; Zhu, Q.; Zhou, Y.; Wang, L. Sample entropy characteristics of movement for four foot types based on plantar centre of pressure during stance phase. Biomed. Eng. Online 2013, 12, 101. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 2002, 89, 068102. [Google Scholar] [CrossRef]
- Humeau-Heurtier, A. The Multiscale Entropy Algorithm and Its Variants: A Review. Entropy 2015, 17, 3110–3123. [Google Scholar] [CrossRef]
- Liao, F.Y.; Cheing, G.L.Y.; Ren, W.Y.; Jain, S.; Jan, Y.K. Application of Multiscale Entropy in Assessing Plantar Skin Blood Flow Dynamics in Diabetics with Peripheral Neuropathy. Entropy 2018, 20, 127. [Google Scholar] [CrossRef]
- Busa, M.A.; van Emmerik, R.E.A. Multiscale entropy: A tool for understanding the complexity of postural control. J. Sport Health Sci. 2016, 5, 44–51. [Google Scholar] [CrossRef]
- Costa, M.; Priplata, A.A.; Lipsitz, L.A.; Wu, Z.; Huang, N.E.; Goldberger, A.L.; Peng, C.K. Noise and poise: Enhancement of postural complexity in the elderly with a stochastic-resonance-based therapy. EPL 2007, 77, 68008. [Google Scholar] [CrossRef]
- Pajala, S.; Era, P.; Koskenvuo, M.; Kaprio, J.; Tormakangas, T.; Rantanen, T. Force platform balance measures as predictors of indoor and outdoor falls in community-dwelling women aged 63-76 years. J. Gerontol. A 2008, 63, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Andrews, A.W. Normal walking speed: A descriptive meta-analysis. Physiotherapy 2011, 97, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Lung, C.W.; Hsiao-Wecksler, E.T.; Bums, S.; Lin, F.; Jan, Y.K. Quantifying Dynamic Changes in Plantar Pressure Gradient in Diabetics with Peripheral Neuropathy. Front. Bioeng. Biotech. 2016, 4, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of biological signals. Phys. Rev. E 2005, 71, 021906. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Earlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Stolwijk, N.M.; Duysens, J.; Louwerens, J.W.; Keijsers, N.L. Plantar pressure changes after long-distance walking. Med. Sci. Sports Exerc. 2010, 42, 2264–2272. [Google Scholar] [CrossRef]
- Thomas, K.S.; VanLunen, B.L.; Morrison, S. Changes in postural sway as a function of prolonged walking. Eur. J. Appl. Physiol. 2013, 113, 497–508. [Google Scholar] [CrossRef]
- Bhatt, T.; Wening, J.D.; Pai, Y.C. Influence of gait speed on stability: Recovery from anterior slips and compensatory stepping. Gait Posture 2005, 21, 146–156. [Google Scholar] [CrossRef]
- McAndrew Young, P.M.; Dingwell, J.B. Voluntarily changing step length or step width affects dynamic stability of human walking. Gait Posture 2012, 35, 472–477. [Google Scholar] [CrossRef] [Green Version]
- Menz, H.B.; Lord, S.R.; Fitzpatrick, R.C. Age-related differences in walking stability. Age Ageing 2003, 32, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Stief, F.; Schafer, A.; Vogt, L.; Kirchner, M.; Hubscher, M.; Thiel, C.; Banzer, W.; Meurer, A. Differences in Gait Performance, Quadriceps Strength, and Physical Activity Between Fallers and Nonfallers in Women with Osteoporosis. J. Aging Phys. Act. 2016, 24, 430–434. [Google Scholar] [CrossRef]
- DeVita, P.; Hortobagyi, T. Age causes a redistribution of joint torques and powers during gait. J. Appl. Physiol. 2000, 88, 1804–1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClymont, J.; Pataky, T.C.; Crompton, R.H.; Savage, R.; Bates, K.T. The nature of functional variability in plantar pressure during a range of controlled walking speeds. R. Soc. Open Sci. 2016, 3, 160369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.L.; Kuo, M.Y.; Chang, C.F.; Lu, T.W.; Hong, S.W. Effects of gait speed on the body’s center of mass motion relative to the center of pressure during over-ground walking. Hum. Mov. Sci. 2017, 54, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.L.; Lu, T.W.; Lin, H.C.; Hsieh, H.J.; Chan, W.P. Effects of belt speed on the body’s center of mass motion relative to the center of pressure during treadmill walking. Gait Posture 2017, 51, 109–115. [Google Scholar] [CrossRef]
- Rosenbaum, D.; Hautmann, S.; Gold, M.; Claes, L. Effects of walking speed on plantar pressure patterns and hindfoot angular motion. Gait Posture 1994, 2, 191–197. [Google Scholar] [CrossRef]
- Segal, A.; Rohr, E.; Orendurff, M.; Shofer, J.; O’Brien, M.; Sangeorzan, B. The effect of walking speed on peak plantar pressure. Foot Ankle Int. 2004, 25, 926–933. [Google Scholar] [CrossRef]
- Chung, M.J.; Wang, M.J. Gender and walking speed effects on plantar pressure distribution for adults aged 20–60 years. Ergonomics 2012, 55, 194–200. [Google Scholar] [CrossRef]
- Burnfield, J.M.; Few, C.D.; Mohamed, O.S.; Perry, J. The influence of walking speed and footwear on plantar pressures in older adults. Clin. Biomech. 2004, 19, 78–84. [Google Scholar] [CrossRef]
- Warren, G.L.; Maher, R.M.; Higbie, E.J. Temporal patterns of plantar pressures and lower-leg muscle activity during walking: Effect of speed. Gait Posture 2004, 19, 91–100. [Google Scholar] [CrossRef]
- Melzer, I.; Benjuya, N.; Kaplanski, J. Postural stability in the elderly: A comparison between fallers and non-fallers. Age Ageing 2004, 33, 602–607. [Google Scholar] [CrossRef] [Green Version]
- Merlo, A.; Zemp, D.; Zanda, E.; Rocchi, S.; Meroni, F.; Tettamanti, M.; Recchia, A.; Lucca, U.; Quadri, P. Postural stability and history of falls in cognitively able older adults: The Canton Ticino study. Gait Posture 2012, 36, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Laughton, C.A.; Slavin, M.; Katdare, K.; Nolan, L.; Bean, J.F.; Kerrigan, D.C.; Phillips, E.; Lipsitz, L.A.; Collins, J.J. Aging, muscle activity, and balance control: Physiologic changes associated with balance impairment. Gait Posture 2003, 18, 101–108. [Google Scholar] [CrossRef]
- Prieto, T.E.; Myklebust, J.B.; Hoffmann, R.G.; Lovett, E.G.; Myklebust, B.M. Measures of postural steadiness: Differences between healthy young and elderly adults. IEEE Trans. Biomed. Eng. 1996, 43, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Xiong, S. Center-of-pressure based postural sway measures: Reliability and ability to distinguish between age, fear of falling and fall history. Int. J. Ind. Ergon. 2015, 47, 37–44. [Google Scholar] [CrossRef]
- Chiu, M.C.; Wu, H.C.; Chang, L.Y. Gait speed and gender effects on center of pressure progression during normal walking. Gait Posture 2013, 37, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Sole, G.; Pataky, T.; Sole, C.C.; Hale, L.; Milosavljevic, S. Age-related plantar centre of pressure trajectory changes during barefoot walking. Gait Posture 2017, 57, 188–192. [Google Scholar] [CrossRef]
- Chiu, M.C.; Wu, H.C.; Chang, L.Y.; Wu, M.H. Center of pressure progression characteristics under the plantar region for elderly adults. Gait Posture 2013, 37, 408–412. [Google Scholar] [CrossRef]
- Hita-Contreras, F.; Martinez-Amat, A.; Lomas-Vega, R.; Alvarez, P.; Mendoza, N.; Romero-Franco, N.; Aranega, A. Relationship of body mass index and body fat distribution with postural balance and risk of falls in Spanish postmenopausal women. Menopause 2013, 20, 202–208. [Google Scholar] [CrossRef]
- Menegoni, F.; Galli, M.; Tacchini, E.; Vismara, L.; Cavigioli, M.; Capodaglio, P. Gender-specific effect of obesity on balance. Obesity 2009, 17, 1951–1956. [Google Scholar] [CrossRef]
- Hue, O.; Simoneau, M.; Marcotte, J.; Berrigan, F.; Dore, J.; Marceau, P.; Marceau, S.; Tremblay, A.; Teasdale, N. Body weight is a strong predictor of postural stability. Gait Posture 2007, 26, 32–38. [Google Scholar] [CrossRef]
- Handrigan, G.; Hue, O.; Simoneau, M.; Corbeil, P.; Marceau, P.; Marceau, S.; Tremblay, A.; Teasdale, N. Weight loss and muscular strength affect static balance control. Int. J. Obes. 2010, 34, 936–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cau, N.; Cimolin, V.; Galli, M.; Precilios, H.; Tacchini, E.; Santovito, C.; Capodaglio, P. Center of pressure displacements during gait initiation in individuals with obesity. J. Neuroeng. Rehabil. 2014, 11, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liau, B.-Y.; Wu, F.-L.; Lung, C.-W.; Zhang, X.; Wang, X.; Jan, Y.-K. Complexity-Based Measures of Postural Sway during Walking at Different Speeds and Durations Using Multiscale Entropy. Entropy 2019, 21, 1128. https://doi.org/10.3390/e21111128
Liau B-Y, Wu F-L, Lung C-W, Zhang X, Wang X, Jan Y-K. Complexity-Based Measures of Postural Sway during Walking at Different Speeds and Durations Using Multiscale Entropy. Entropy. 2019; 21(11):1128. https://doi.org/10.3390/e21111128
Chicago/Turabian StyleLiau, Ben-Yi, Fu-Lien Wu, Chi-Wen Lung, Xueyan Zhang, Xiaoling Wang, and Yih-Kuen Jan. 2019. "Complexity-Based Measures of Postural Sway during Walking at Different Speeds and Durations Using Multiscale Entropy" Entropy 21, no. 11: 1128. https://doi.org/10.3390/e21111128






