Energy Use of Flux Salt Recovery Using Bipolar Membrane Electrodialysis for a CO2 Mineralisation Process
Abstract
:1. Introduction
Bipolar Membranes (BPM)
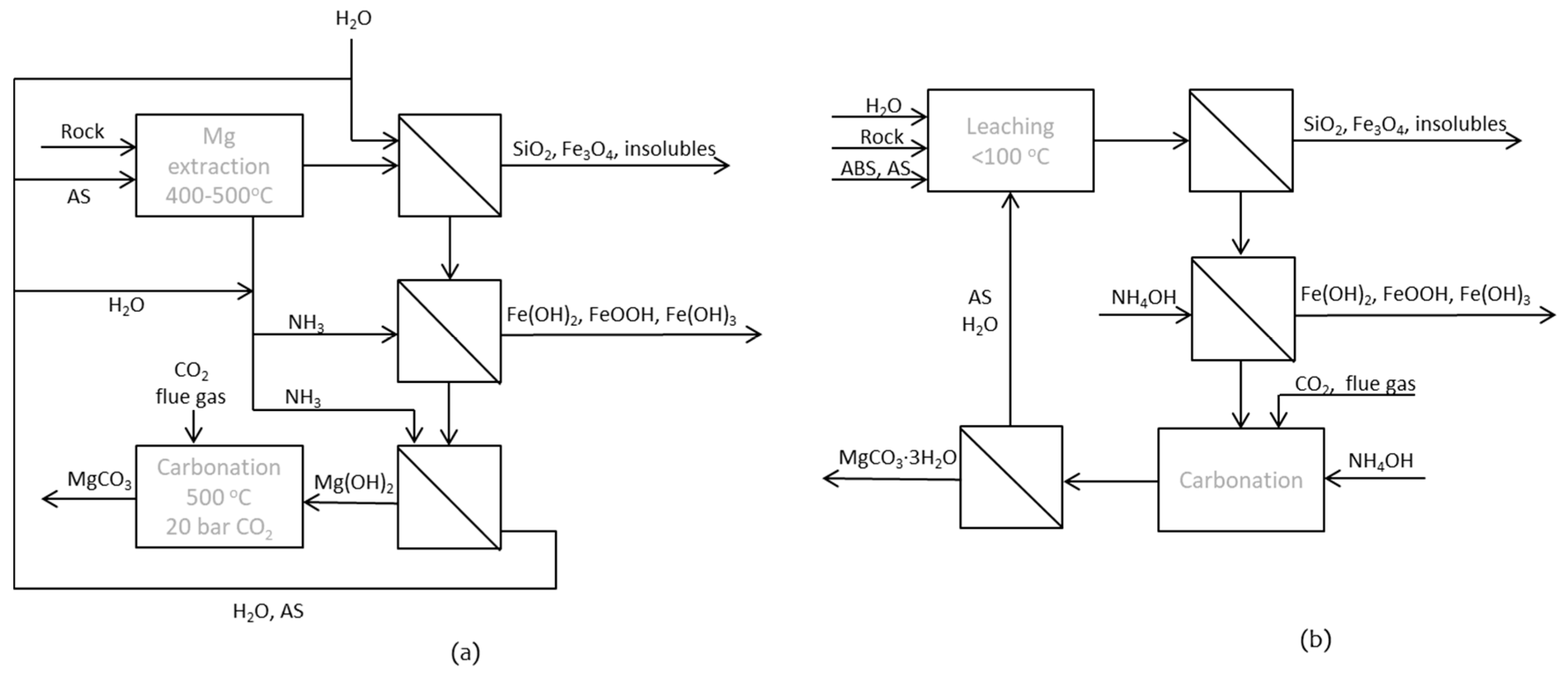
2. Materials and Methods
2.1. Exergy Calculations and Calculations of the Energy Need for Separating 1 kg of Ammonium
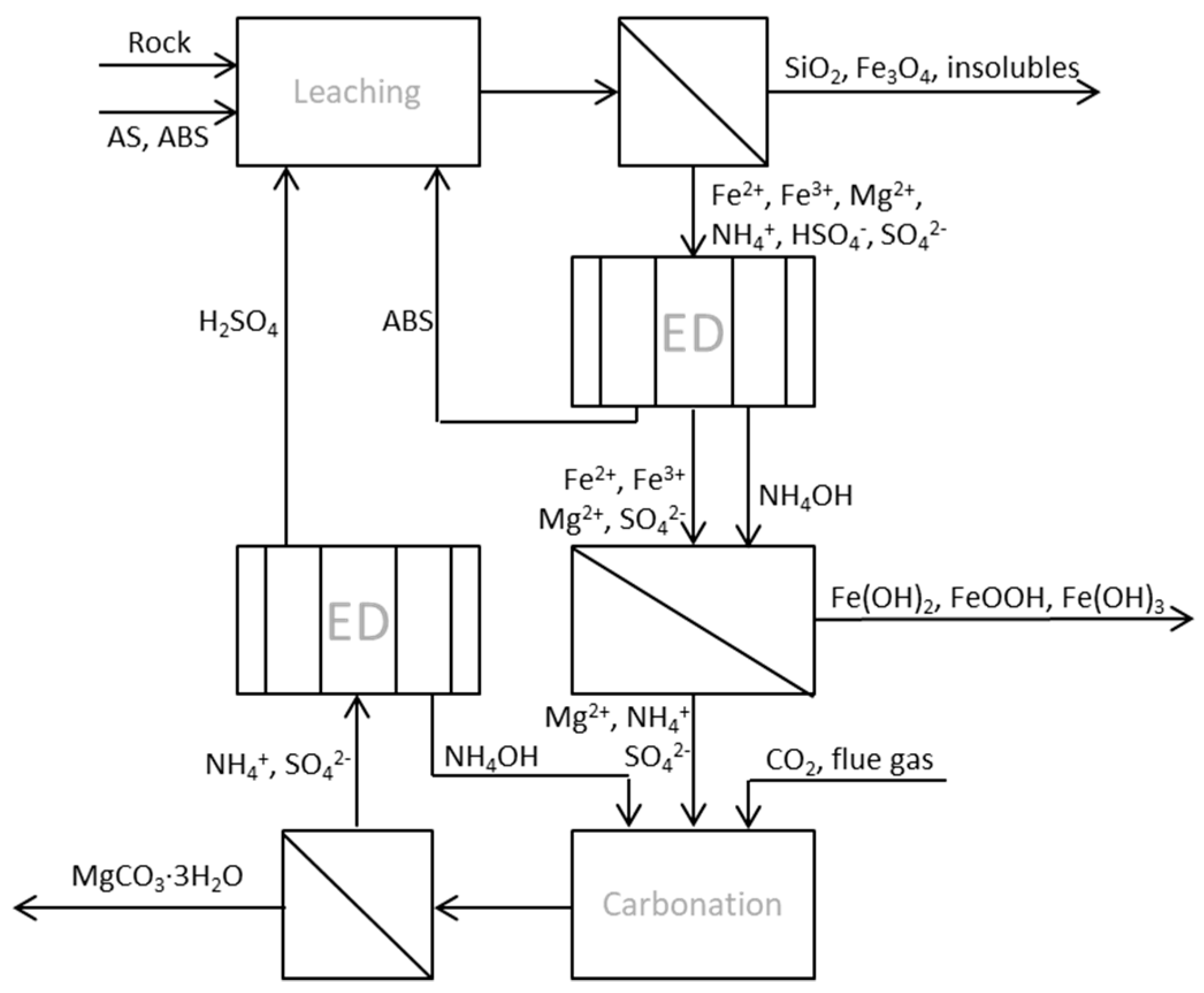
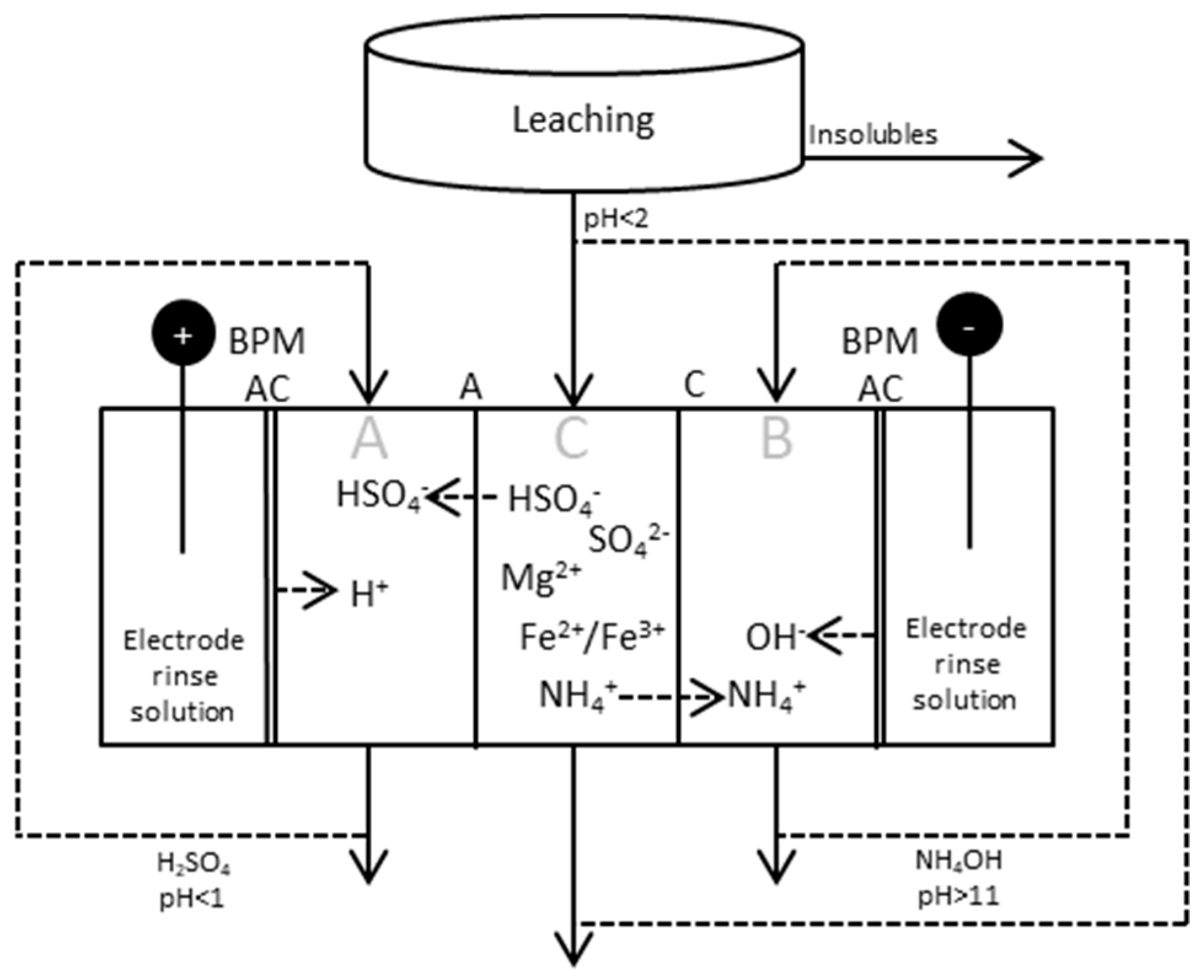
2.2. BPMED after Leaching Step
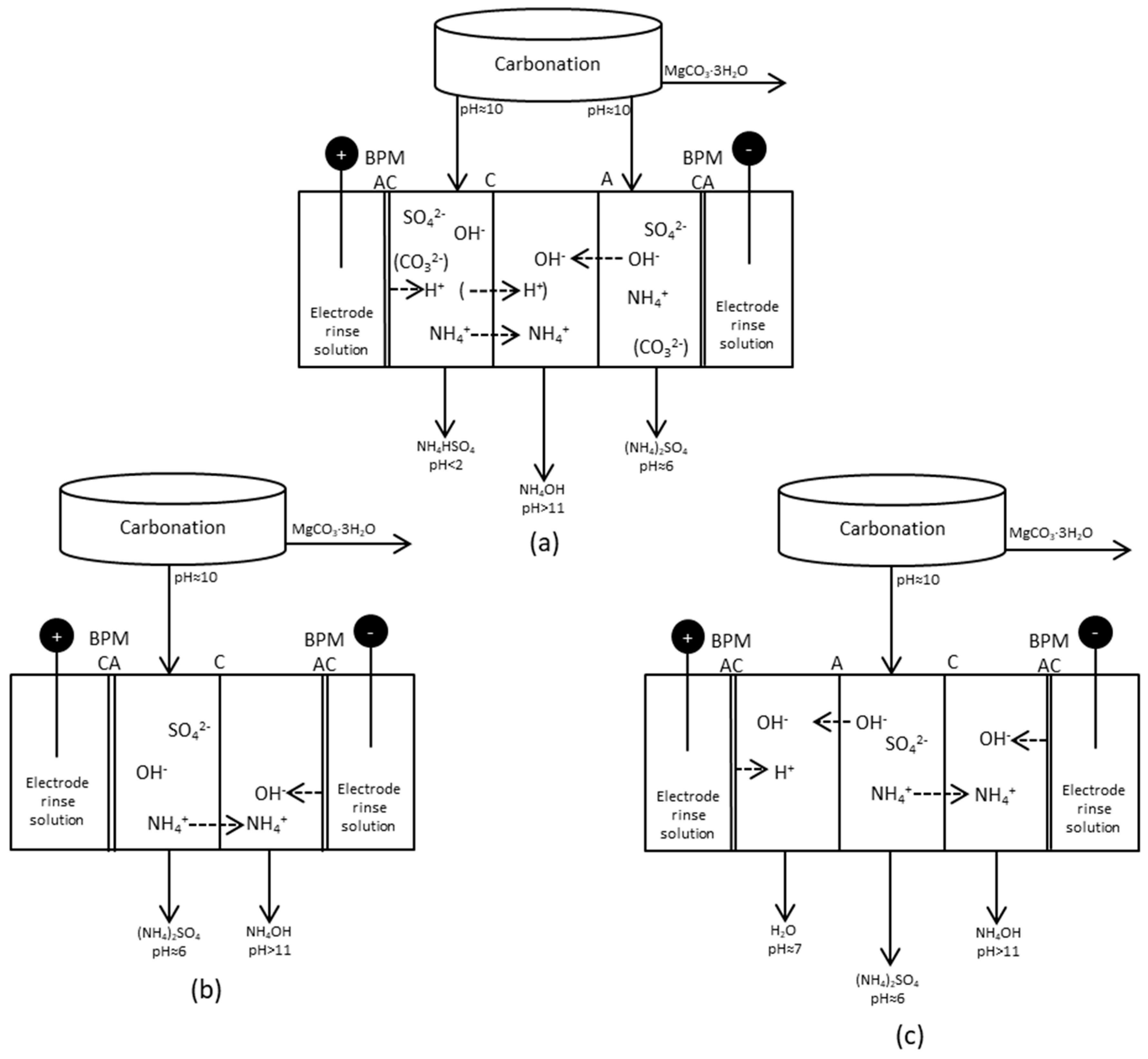
2.3. BPMED after Carbonation Step
3. Results and Discussion
3.1. BPMED after Leaching
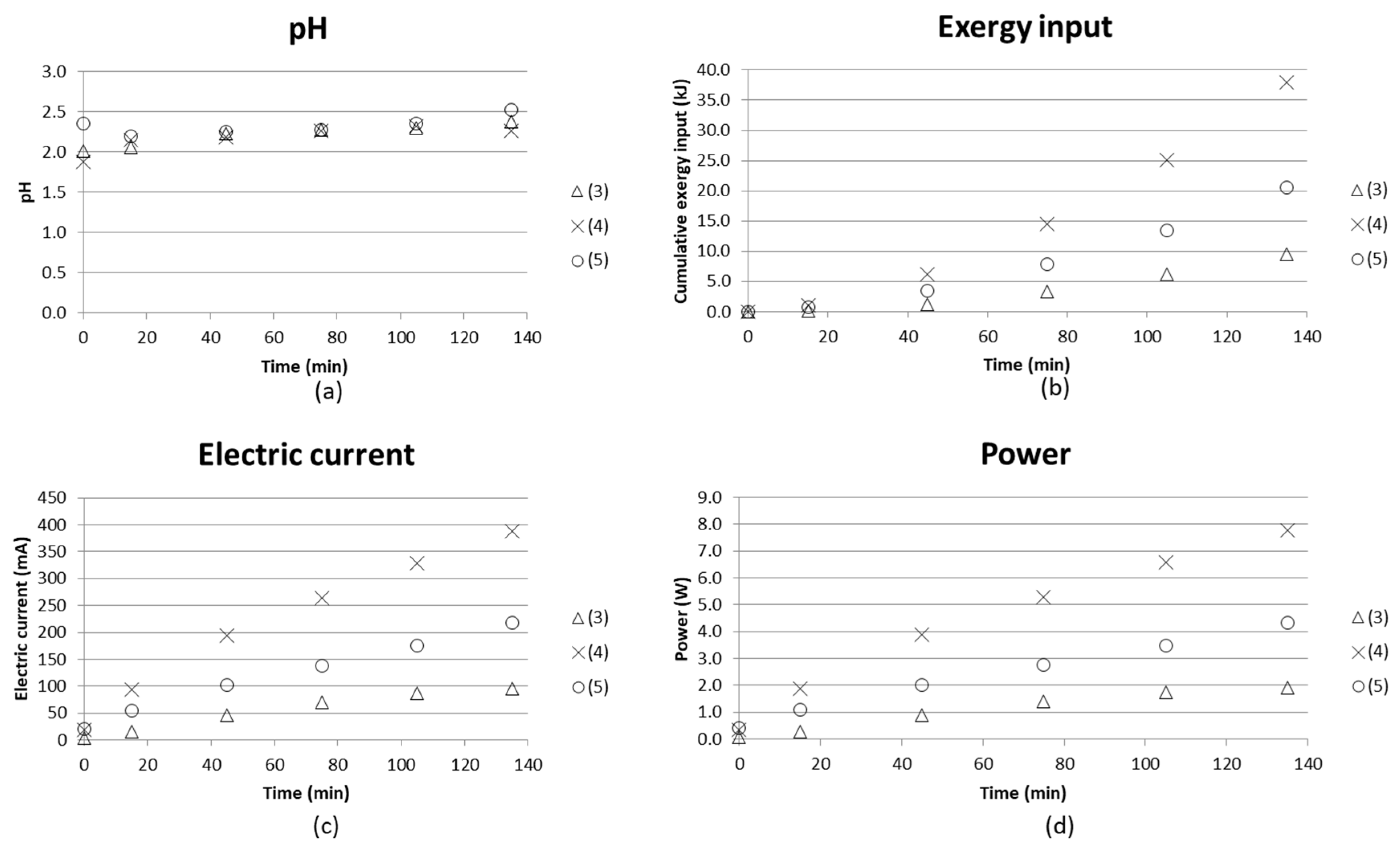
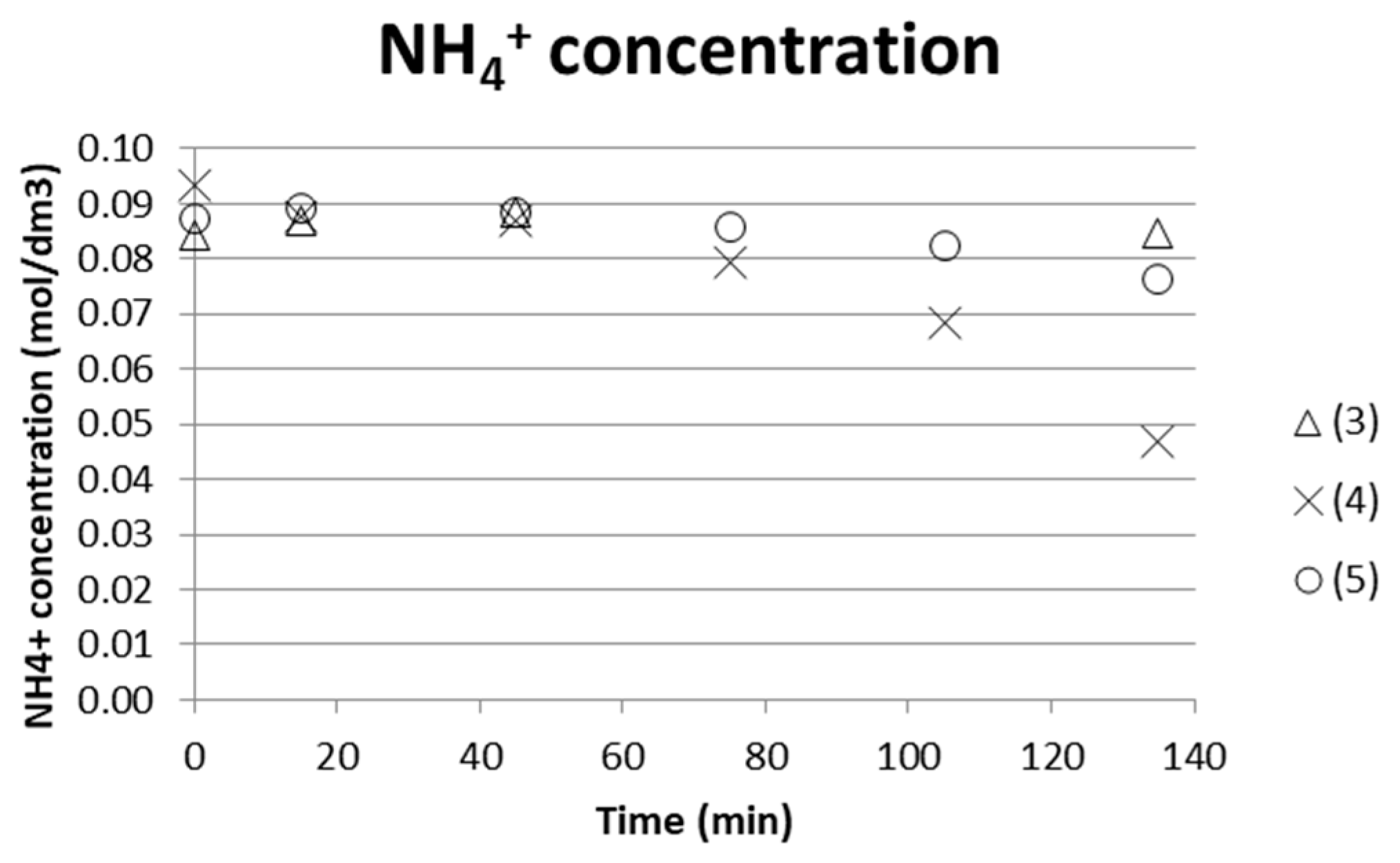
3.1.1. Initial BPMED Batch Mode Tests (Tests (3)–(5))
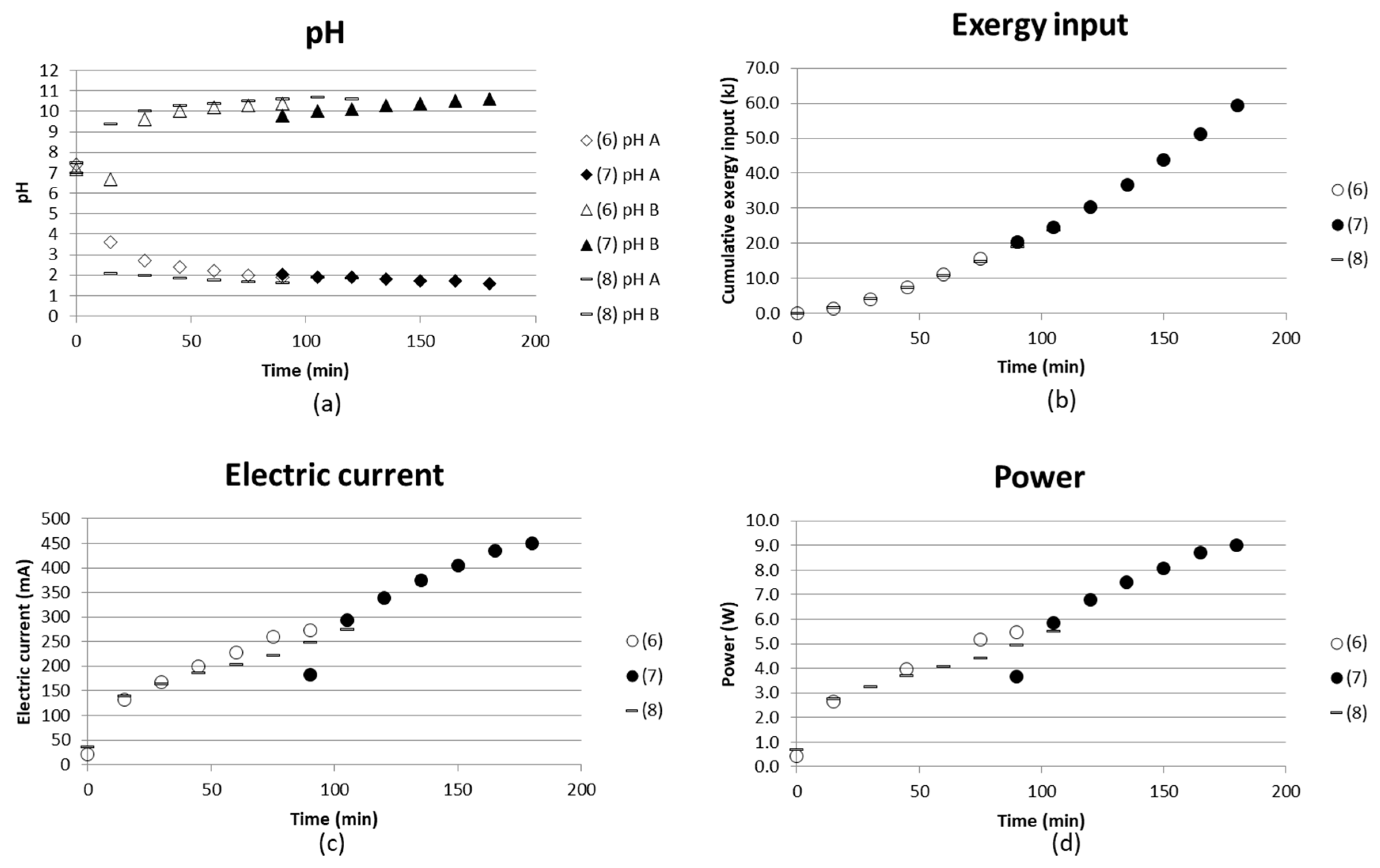
3.1.2. Interruption and Recirculation Instead of Continuous Flow (Tests (6)–(8))
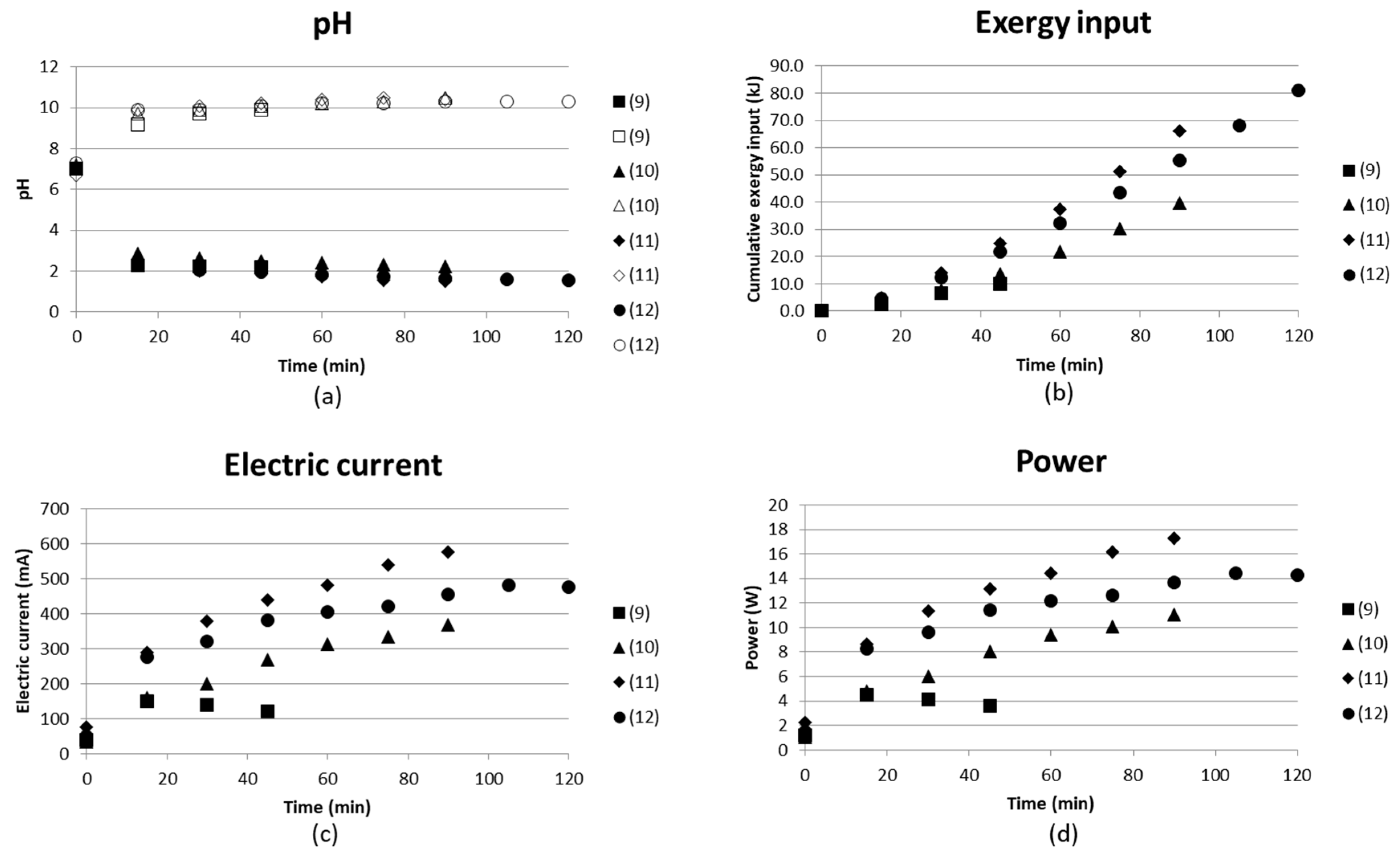
3.1.3. Continuous Flow Tests at 30 V (Tests (9)–(12))
3MgSO4(aq) + 2SiO2(s) + 6NH3(aq)+ 5H2O(aq)
3MgSO4 (aq) + 2SiO2 (s) + 5H2O (aq)+ 3(NH4)2SO4(aq)
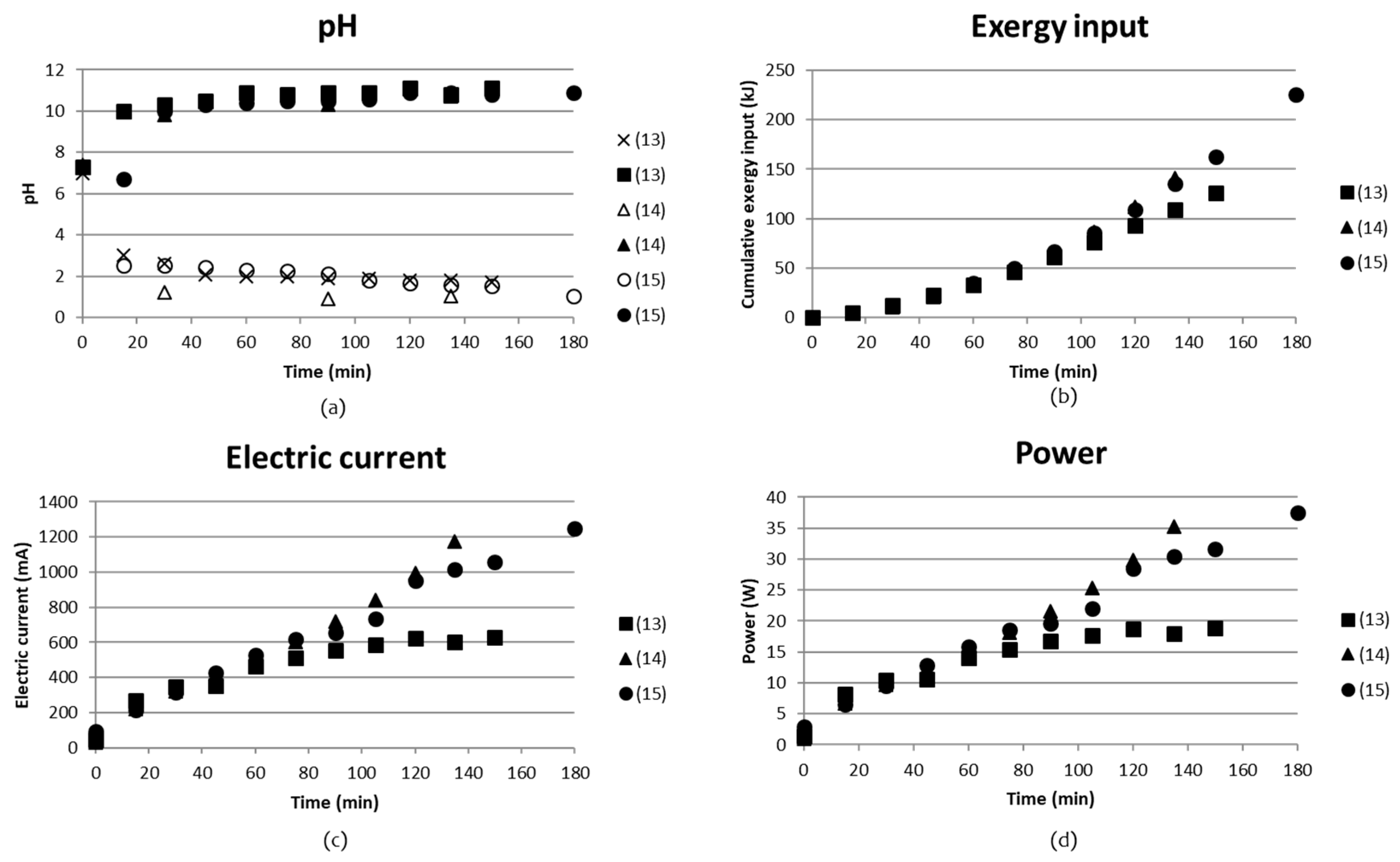
3.1.4. BPMED with Rock-Derived Solutions (Tests (13)–(15))
3.2. BPMED after Carbonation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ÅA | Åbo Akademi University |
| A | acidic solution (in Figure 3, Figure 5 and Figure 6 indicating also anion membrane) |
| ABS | ammonium bisulfate, NH4HSO4 |
| ACS | monovalent anion exchange membrane |
| AS | ammonium sulfate, (NH4)2SO4 |
| B | basic solution |
| BPM | bipolar membrane |
| BPMED | bipolar membrane electrodialysis |
| C | concentrate (in Figure 3, Figure 5 and Figure 6 indicating also cation membrane) |
| CMS | monovalent cation exchange membrane |
| ED | electrodialysis |
| ISE | ion selective electrode |
References
- Official Statistics of Finland (OSF). Greenhouse Gases [e-Publication]; Statistics Finland: Helsinki, Finland, 2017; Available online: http://www.stat.fi/til/khki/2017/khki_2017_2018-12-11_tie_001_en.html (accessed on 11 February 2019).
- Ministry of the Environment. National Climate Change Policy. Available online: http://www.ym.fi/en-US/The_environment/Climate_and_air/Mitigation_of_climate_change/National_climate_policy (accessed on 11 February 2019).
- Ministry of Economic Affairs and Employment of Finland. Finland’s New Energy and Climate Strategy. Available online: https://tem.fi/en/energy-and-climate-strategy-2016 (accessed on 11 February 2019).
- Nduagu, E. Production of Mg(OH)2 from Mg-Silicate Rock for CO2 Sequestration. Ph.D. Thesis, Åbo Akademi University, Turku, Finland, 2012. [Google Scholar]
- Fagerlund, J. Carbonation of Mg(OH)2 in a Pressurised Fluidised Bed for CO2 Sequestration. Ph.D. Thesis, Åbo Akademi University, Turku, Finland, 2012. [Google Scholar]
- Romão, I.S. Production of Magnesium Carbonates from Serpentinites for CO2 Mineral Sequestration-Optimisation towards Industrial Application. Ph.D. Thesis, Åbo Akademi University/University of Coimbra, Turku, Finland; Coimbra, Portugal, 2015. [Google Scholar]
- Slotte, M. Two Process Case Studies on Energy Efficiency, Life Cycle Assessment and Process Scale-Up. Ph.D. Thesis, Åbo Akademi University, Turku, Finland, 2017. [Google Scholar]
- Lavikko, S. Geological and Mineralogical Aspects on Mineral Carbonation. Ph.D. Thesis, Åbo Akademi University, Turku, Finland, 2017. [Google Scholar]
- Wang, X.; Maroto-Valer, M.M. Dissolution of serpentinite using ammonium salts for CO2 mineral carbonation. Fuel 2011, 90, 1229–1237. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Slotte, M.; Åbacka, J.; Highfield, J. A comparison of CO2 mineral carbonation processes involving a dry or a wet carbonation step. Energy 2016, 117, 604–611. [Google Scholar] [CrossRef]
- Erlund, R.; Koivisto, E.; Fagerholm, M.; Zevenhoven, R. Extraction of magnesium from four Finnish magnesium silicate rocks for CO2 mineralisation—Part 2: Aqueous solution extraction. Hydrometallurgy 2016, 166, 229–236. [Google Scholar] [CrossRef]
- Romão, I.S.; Gando-Ferreira, L.M.; Zevenhoven, R. Combined extraction of metals and production of Mg(OH)2 for CO2 sequestration from nickel mine ore overburden. Miner. Eng. 2013, 53, 167–170. [Google Scholar] [CrossRef]
- Koivisto, E.; Zevenhoven, R. Methods for recovery and re-use of additive chemicals during CO2 mineralisation. J. Water Proc. Eng. 2017, 20, 61–70. [Google Scholar] [CrossRef]
- Sanna, A.; Gaubert, J.; Maroto-Valer, M.M. Alternative regeneration of chemicals employed in mineral carbonation towards technology cost reduction. Chem. Eng. J. 2016, 306, 1049–1057. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, W.; Ren, H.; Cong, W. Sulfuric acid ammonia generation by bipolar membranes electrodialysis: Transport rate model for ion and water through anion exchange membrane. Chem. Biochem. Eng. 2008, 22, 1–8. [Google Scholar]
- Koivisto, E.; Zevenhoven, R. Membrane separation of ammonium bisulfate from ammonium sulfate in aqueous solutions for CO2 mineralisation. Geosciences 2018, 8, 123. [Google Scholar] [CrossRef]
- Romão, I.S.; Eriksson, M.; Nduagu, E.; Fagerlund, J.; Gando-Ferreira, L.M.; Zevenhoven, R. Carbon dioxide storage by mineralisation applied to a lime kiln. In Proceedings of the ECOS 2012, Perugia, Italy, 26–29 June 2012. p. 226. [Google Scholar]
- Slotte, M.; Romão, I.; Zevenhoven, R. Integration of a pilot-scale serpentinite carbonation process with an industrial lime kiln. Energy 2013, 62, 142–149. [Google Scholar] [CrossRef]
- Slotte, M.; Zevenhoven, R. Total lime kiln gas compression for CO2 mineral sequestration. In Proceedings of the ECOS 2013, Guilin, China, 15–19 July 2013. p. F005. [Google Scholar]
- Zevenhoven, R.; Fagerlund, J.; Nduagu, E.; Romão, I.; Jie, B.; Highfield, J. Carbon storage by mineralisation (CSM): Serpentinite rock carbonation via Mg(OH)2 reaction intermediate without CO2 pre-separation. Energy Procedia 2013, 37, 5945–5954. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Slotte, M.; Koivisto, E.; Erlund, R. Serpentinite Carbonation Process Routes using Ammonium Sulfate and Integration in Industry. Energy Technol. 2017, 5, 945–954. [Google Scholar] [CrossRef]
- Mani, K.N. Electrodialysis water splitting technology. J. Membr. Sci. 1991, 58, 117–138. [Google Scholar] [CrossRef]
- Frilette, V.J. Preparation and Characterization of Bipolar Ion Exchange Membranes. J. Phys. Chem. 1956, 60, 435–439. [Google Scholar] [CrossRef]
- Arribas, P.; Khayet, M.; Garcia-Payo, M.C.; Gil, L. Novel and emerging membranes for water treatment by electroc potential and concentration gradient membrane processes. In Advances in Membrane Technologies for Water Treatment, 1st ed.; Basile, A., Cassano, A., Rastogi, N.K., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 287–325. [Google Scholar]
- Van der Bruggen, B. Advances in electrodialysis for water treatment In Advances in Membrane Technologies for Water Treatment, 1st ed.; Basile, A., Cassano, A., Rastogi, N.K., Eds.; Woodhead Publishing: Cambridge, United Kingdom, 2015; pp. 185–203. [Google Scholar]
- Gineste, J.L.; Pourcelly, G.; Lorrain, Y.; Persin, F.; Gavach, C. Analysis of factors limiting the use of bipolar membranes: A simplified model to determine trends. J. Membr. Sci. 1996, 112, 199–208. [Google Scholar] [CrossRef]
- Eurodia. Available online: https://www.eurodia.com/en/ (accessed on 27 February 2019).
- Szargut, J. Exergy Method: Technical and Ecological Applications; Publishing: Gateshead, UK, 2005. [Google Scholar]
- Wang, Q.; Yang, P.; Cong, W. Cation-exchange membrane fouling and cleaning in bipolar membrane electrodialysis of industrial glutamate production wastewater. Sep. Purif. Technol. 2011, 79, 103–113. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, C.; Pi, K.; Huang, J.; Xia, M.; Gerson, A.R. Sustainable treatment of desulfurization wastewater by ion exchange and bipolar membrane electrodialysis hybrid technology. Sep. Purif. Technol. 2019, 211, 330–339. [Google Scholar] [CrossRef]
- Tongwen, X. Electrodialysis processes with bipolar membranes (EDBM) in environmental protection—A review. Resour. Conserv. Recycl. 2002, 37, 1–22. [Google Scholar] [CrossRef]
- Nico 2000. Available online: http://www.nico2000.net/analytical/ammonium.htm (accessed on 27 February 2019).
- Vermaas, D.A.; Wiegman, S.; Nagakia, T.; Smith, W.A. Ion transport mechanisms in bipolar membranes for (photo)electrochemical water splitting. Sustain. Energ. Fuels 2018, 2, 2006–2015. [Google Scholar] [CrossRef]



| Compound | %-wt, Dry |
|---|---|
| CaO | 0.4 |
| SiO2 | 35.5 |
| TiO2 | 0.04 |
| NiO | 0.35 |
| Al2O3 | 0.45 |
| Fe2O3 | 14.6 |
| MgO | 35.1 |
| K2O | 0.02 |
| Na2O | <0.01 |
| MnO | 0.12 |
| CuO | 0.10 |
| Cr2O3 | 0.38 |
| P2O5 | 0.01 |
| S-Eltra | 0.42 |
| LOI 1000 °C | 12.0 |
| Rest | 0.5 |
| Test | Synthetic or Rock-Derived | Rinse Solution | Voltage (V) | Total Time (min) | Mode (Recirc./Batch/Cont.) Flow Rate Conc. Compartment | Mode (Recirc./Batch/Cont.) Flow Rate Acid + Base Compartments |
|---|---|---|---|---|---|---|
| (1) * | Synthetic | H2O | 20 | 135 | Batch | Batch |
| (2) * | Synthetic | H2O | 30 | 135 | Batch | Batch |
| (3) | Synthetic | H2O | 20 | 135 | Batch | Batch |
| (4) | Synthetic | 0.05/0.05 | 20 | 135 | Batch | Batch |
| (5) | Synthetic | AS/ABS 0.05/0.05 | 20 | 135 | Batch | Batch |
| (6) | Synthetic | AS/ABS 0.05/0.05 | 20 | 90 | Recirc. 1 L/h | Recirc. 1 L/h |
| (7) | Synthetic | AS/ABS 0.05/0.05 | 20 | 90 | Recirc. 1 L/h | Recirc. 1L/h |
| (8) | Synthetic | AS/ABS 0.05/0.05 | 20 | 105 | Cont. 1 L/h | Recirc. 1 L/h |
| (9) | Synthetic | AS/ABS 0.05/0.05 | 30 | 53 | Cont. 1.14 L/h | Cont. 1.14 L/h |
| (10) | Synthetic | AS/ABS 0.05/0.05 | 30 | 120 | Cont. 0.56 L/h | Cont. 0.64 + 0.62 L/h |
| (11) | Synthetic | AS/ABS 0.05/0.05 | 30 | 90 | Cont. 0.67 L/h | Cont. 0.33 L/h |
| (12) | Synthetic | AS/ABS 0.05/0.05 | 30 | 120 | Cont. 0.25 L/h | Cont. 0.2 L/h |
| (13) | Rock der. | AS/ABS 0.05/0.05 | 30 | 143 | Cont. 0.25 L/h | Cont. 0.275 L/h |
| (14) | Rock der. | 0.15 M AS | 30 | 135 | Batch | Batch |
| (15) | Rock der. | 0.15 M AS | 30 | 180 | Cont. 0.25 L/h | Recirc. 0.084 L/h |
| Composition of Starting Solution | |||||
|---|---|---|---|---|---|
| Test | ABS | AS | MgSO4 | Fe(II)SO4∙7H2O | Fe(III)2(SO4)3∙H2O |
| (1) | 0.05 M | 0.05M | - | - | - |
| (2) | 0.05 M | 0.05 M | - | - | - |
| (3) | 0.05 M | 0.05 M | - | - | - |
| (4) | 0.05 M | 0.05 M | - | - | - |
| (5) | 0.05 M | 0.05 M | 0.03 M | 0.01 M | - |
| (6) | 0.05 M | 0.05 M | 0.03 M | 0.01 M | - |
| (7) | 0.05 M | 0.05 M | 0.03 M | 0.01 M | - |
| (8) | 0.05 M | 0.05 M | 0.03 M | 0.01 M | - |
| (9) | 0.54 M | 0.7 M | 0.16 M | 0.009 M | 0.0015 M |
| (10) | 0.54 M | 0.7 M | 0.16 M | 0.009 M | 0.0015 M |
| (11) | 0.54 M | 0.7 M | 0.16 M | 0.009 M | 0.0015 M |
| (12) | C(out) from test (10) | ||||
| (13) | Solution from leaching: Hitura, 63–125 µm, 70 °C, 0.8 L, 20 g | ||||
| (14) | C(out) from test (13) | ||||
| (15) | Solution from leaching: Hitura, 63–125 µm, 70 °C, 0.8 L, 20 g | ||||
| Test | Setup (Figure 6) | Number of Compartments | Initial AS Solution * | Membrane Order |
|---|---|---|---|---|
| 1 | (a) | 5 | 1.0 M | +BPM(ACS/CMS)-CMS-ACS-BPM(CMS/ACS)- |
| 2 | (a) | 5 | 0.15 M | +BPM(ACS/CMS)-CMS-ACS-BPM(CMS/ACS)- |
| 3 | (b) | 4 | 0.15 M | +BPM(CMS/ACS)-CMS-BPM(ACS/CMS)- |
| 4 | (c) | 5 | 0.15 M | +BPM(ACS/CMS)-ACS-CMS-BPM(ACS/CMS)- |
| Test Number | (9) | (10) | (11) | (12) | H2O in |
|---|---|---|---|---|---|
| Conductivitystop (µS/cm) | 70 | 140 | 420 | 295 | 30 |
| pH | 9.9 | 10.4 | 10.4 | 10.3 | 7 |
| Test | Sample Type | Comp. No. | Vol. Tot. (L) | SO42− (g/L) | NH4+ (g/L) | Mg2+ (g/L) | Fe2+/3+ (g/L) | Ni+/2+ (g/L) | pH |
|---|---|---|---|---|---|---|---|---|---|
| 13 | Cin | Comp. 2 | 130 | 2.9 | 0.69 | 0.027 | 1.8 | ||
| 13 | Aout | Comp. 1 | 0.8 | 2.3 | 1.7 | ||||
| 13 | Cout | Comp. 2 | 0.75 | 130 | 37 | 2.8 | 0.68 | 0.027 | 1.8 |
| 13 | Bout | Comp. 3 | 0.8 | 0.43 | 11.1 | ||||
| 14 | Cin = Cout(13) | Comp. 2 | 130 | 2.8 | 0.68 | 0.027 | 1.5 | ||
| 14 | Aout | Comp. 1 | 0.135 | 14 | 1.0 | ||||
| 14 | Cout | Comp. 2 | 0.135 | 110 | 31 | 2.7 | 0.66 | 0.026 | 1.8 |
| 14 | Bout | Comp. 3 | 0.135 | 1.7 | 10.7 | ||||
| 15 | Cin | Comp. 2 | 130 | 2.7 | 0.64 | 0.026 | 1.9 | ||
| 15 | Aout | Comp. 1 | 0.2 | 15 | 1.0 | ||||
| 15 | Cout | Comp. 2 | 0.75 | 130 | 35 | 2.7 | 0.66 | 0.026 | 1.7 |
| 15 | Bout | Comp. 3 | 0.2 | 3.9 | 10.9 | ||||
| CL2 | 14 | 2.2 | 0.29 | 0.021 | 2.3 |
| Setup | Concentration in (M) | Compartment | SO42− in (%) | NH4+ in (%) | SO42− out (%) | NH4+ out (%) | pH out |
|---|---|---|---|---|---|---|---|
| (a) | 1.0 AS+ NH4OH to pH 10 | Comp. 1 | 50 | 50 | 46.0 | 46.3 | 9.2 |
| (a) | Dest. H2O | Comp. 2 NH4OH | 0 | 0 | 14.9 | 16.5 | 9.7 |
| (a) | 1.0 AS+ NH4OH to pH 10 | Comp. 3 AS | 50 | 50 | 39.1 | 37.2 | 9.6 |
| SUM | 100 | 100 | 100 | 100 | |||
| (a) | 0.15 AS+ NH4OH to pH 10 | Comp. 1 | 50 | 50 | 51.6 | 35.3 | 8.9 |
| (a) | Dest. H2O | Comp. 2 NH4OH | 0 | 0 | 45.2 | 47.1 | 9.4 |
| (a) | 0.15 AS+ NH4OH to pH 10 | Comp. 3 | 50 | 50 | 3.2 | 17.6 | 10.4 |
| SUM | 100 | 100 | 100 | 100 | |||
| (b) | 0.15 AS+ NH4OH to pH 10 | Comp. 1 | 100 | 100 | 99.2 | 60.0 | 9.8 |
| (b) | Dest. H2O | Comp. 2 NH4OH | 0 | 0 | 0.8 | 40.0 | 11.3 |
| SUM | 100 | 100 | 100 | 100 | |||
| (c) | Dest. H2O | Comp. 1 | 0 | 0 | 76.6 | 6.5 | 1.2 |
| (c) | 0.15 AS+ NH4OH to pH 10 | Comp. 2 | 100 | 100 | 23.0 | 51.6 | 10.2 |
| (c) | Dest. H2O | Comp. 3 NH4OH | 0 | 0 | 0.4 | 41.9 | 11.2 |
| SUM | 100 | 100 | 100 | 100 |
| Test | Total Volume (L) | Total Time (min) | pH in Solution Analyzed | Analysis Method | Analysis from Sol. | Synthetic or Rock-Derived | MJ/kg NH4+ |
|---|---|---|---|---|---|---|---|
| (4) | 0.135 | 135 | 2.2 | ISE | Conc. sol. | Synth. | 350 |
| (11) | 0.5 | 90 | 10.4 | External | Basic sol. | Rock | 302 |
| (15) | 0.2 | 180 | 10.9 | External | Basic sol. | Synth. | 3.4 |
| (c) | 0.135 | 135 | 11.2 | External | Basic sol. | Synth. | 1.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koivisto, E.; Zevenhoven, R. Energy Use of Flux Salt Recovery Using Bipolar Membrane Electrodialysis for a CO2 Mineralisation Process. Entropy 2019, 21, 395. https://doi.org/10.3390/e21040395
Koivisto E, Zevenhoven R. Energy Use of Flux Salt Recovery Using Bipolar Membrane Electrodialysis for a CO2 Mineralisation Process. Entropy. 2019; 21(4):395. https://doi.org/10.3390/e21040395
Chicago/Turabian StyleKoivisto, Evelina, and Ron Zevenhoven. 2019. "Energy Use of Flux Salt Recovery Using Bipolar Membrane Electrodialysis for a CO2 Mineralisation Process" Entropy 21, no. 4: 395. https://doi.org/10.3390/e21040395




