Application of the Variance Delay Fuzzy Approximate Entropy for Autonomic Nervous System Fluctuation Analysis in Obstructive Sleep Apnea Patients
Abstract
:1. Introduction
2. Methods
2.1. Data
2.2. HRV Analysis Method
2.2.1. Preprocess
2.2.2. Frequency Domain Analysis
2.2.3. Complexity Analysis
2.3. Simulation Test
2.4. Indices Validation
3. Results
3.1. Simulation Results Comparisons of Indices
3.2. Disease Severity Analisis of OSA
3.3. OSA Screening
3.4. Analysis of Different Scales for RR Intervals
4. Discussion
4.1. Comparison and Summary
4.2. Method Proposed and Parameter Selection
4.3. Physiological Significance
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gilman, M.P.; Floras, J.S.; Kengo, U.; Yasuyuki, K.; Leung, R.S.T.; Douglas, B.T. Continuous positive airway pressure increases heart rate variability in heart failure patients with obstructive sleep apnoea. Clin. Sci. 2008, 114, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Bolea, J.; Pueyo, E.; Orini, M.; Bailon, R. Influence of heart rate in non-linear HRV indices as a sampling rate effect evaluated on supine and standing. Front. Physiol. 2016, 7, 501. [Google Scholar] [CrossRef]
- Olmetti, F.; Rovere, M.T.L.; Robbi, E.; Taurino, A.E.; Fanfulla, F. Nocturnal cardiac arrhythmia in patients with obstructive sleep apnea. Sleep Med. 2008, 9, 475–480. [Google Scholar] [CrossRef]
- Sharma, M.; Agarwal, S.; Acharya, U.R. Application of an optimal class of antisymmetric wavelet filter banks for obstructive sleep apnea diagnosis using ECG signals. Comput. Biol. Med. 2018, 100, 100–113. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.; Xie, H.; Yu, W. Characterization of surface EMG signal based on fuzzy entropy. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 266. [Google Scholar] [CrossRef]
- Cirugeda-Roldan, E.; Cuesta-Frau, D.; Miro-Martinez, P.; Oltra-Crespo, S. Comparative study of entropy sensitivity to missing biosignal data. Entropy 2014, 16, 5901–5918. [Google Scholar] [CrossRef]
- Trimer, R.; Mendes, R.G.; Costa, F.S.; Sampaio, L.M.; Delfino, A., Jr.; Arena, R.; Aletti, F.; Ferrario, M.; Borghi-Silva, A. Is there a chronic sleep stage-dependent linear and nonlinear cardiac autonomic impairment in obstructive sleep apnea? Sleep Breath. 2014, 18, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Gula, L.J.; Krahn, A.D.; Skanes, A.; Ferguson, K.A.; George, C.; Yee, R.; Klein, G.J. Heart rate variability in obstructive sleep apnea: A prospective study and frequency domain analysis. Ann. Noninvasive Electrocardiol. 2015, 8, 144–149. [Google Scholar] [CrossRef]
- Khoo, M.C.K.; Kim, T.-S.; Berry, R.B. Spectral indices of cardiac autonomic function in obstructive sleep apnea. Sleep 1999, 22, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Luz, G.C.D.; Julio, C.S.; Paloma, D.B.S.; Elisabete, A.S.M.; Ramona, C.; Audrey, B.S.; Renata, T.; da Silva, A.L.G. Respiratory muscle strength effect on linear and nonlinear heart rate variability parameters in COPD patients. Int. J. COPD 2016, 11, 1671–1677. [Google Scholar]
- Brennan, M.; Palaniswami, M.; Kamen, P. Do existing measures of Poincare plot geometry reflect nonlinear features of heart rate variability? IEEE Trans. Biomed. Eng. 2001, 48, 1342–1347. [Google Scholar] [CrossRef]
- Carvajal, R.; Wessel, N.; Vallverdú, M.; Caminal, P.; Voss, A. Correlation dimension analysis of heart rate variability in patients with dilated cardiomyopathy. Comput. Methods Programs Biomed. 2005, 78, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Khandoker, A.H.; Jelinek, H.F.; Palaniswami, M. Identifying diabetic patients with cardiac autonomic neuropathy by heart rate complexity analysis. Biomed. Eng. Online 2009, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkiomaki, J.S. Heart rate variability and non-linear dynamics in risk stratification. Front. Physiol. 2011, 2, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.K.; Ha, J.H.; Ryu, S.H.; Yu, J.; Park, D.H. The effect of aging and severity of sleep apnea on heart rate variability indices in obstructive sleep apnea syndrome. Psychiatry Investig. 2012, 9, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbanik, D.; Gac, P.; Martynowicz, H.; Poreba, M.; Podgorski, M.; Negrusz-Kawecka, M.; Mazur, G.; Sobieszczanska, M.; Poreba, R. Obstructive sleep apnea as a predictor of reduced heart rate variability. Sleep Med. 2019, 54, 8–15. [Google Scholar] [CrossRef]
- Doo-Heum, P.; Chul-Jin, S.; Seok-Chan, H.; Jaehak, Y.; Seung-Ho, R.; Eui-Joong, K.; Hong-Beom, S.; Byoung-Hak, S. Correlation between the severity of obstructive sleep apnea and heart rate variability indices. J. Korean Med. Sci. 2008, 23, 226–231. [Google Scholar]
- Porta, A.; Guzzetti, S.; Montano, N.; Furlan, R.; Pagani, M.; Malliani, A.; Cerutti, S. Entropy, entropy rate, and pattern classification as tools to typify complexity in short heart period variability series. IEEE Trans. Biomed. Eng. 2001, 48, 1282–1291. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wei, S.; Long, Y.; Liu, C. Performance analysis of multiscale entropy for the assessment of ECG signal quality. J. Electr. Comput. Eng. 2015, 2015, 563915. [Google Scholar] [CrossRef] [Green Version]
- Al-Angari, H.M.; Sahakian, A.V. Use of sample entropy approach to study heart rate variability in obstructive sleep apnea syndrome. IEEE Trans. Biomed. Eng. 2007, 54, 1900–1904. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.-Y.; Su, M.-C.; Wu, H.-T.; Lin, M.-C.; Tsai, I.T.; Sun, C.-K. Multiscale entropy analysis of heart rate variability for assessing the severity of sleep disordered breathing. Entropy 2015, 17, 231–243. [Google Scholar] [CrossRef]
- Ravelo-García, A.; Navarro-Mesa, J.; Casanova-Blancas, U.; Martin-Gonzalez, S.; Quintana-Morales, P.; Guerra-Moreno, I.; Canino-Rodríguez, J.; Hernández-Pérez, E. Application of the permutation entropy over the heart rate variability for the improvement of electrocardiogram-based sleep breathing pause detection. Entropy 2015, 17, 914–927. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.Y.; Liu, G.; Zhou, G.; Wang, Q.; Chen, S. Robustness evaluation of heart rate variability measures for age gender related autonomic changes in healthy volunteers. Australas. Phys. Eng. Sci. Med. 2014. [Google Scholar] [CrossRef]
- Fujimoto, K.; Ura, M.; Yamazaki, H.; Uematsu, A. Instability of parasympathetic nerve function evaluated by instantaneous time–frequency analysis in patients with obstructive sleep apnea. Sleep Biol. Rhythm. 2018, 16, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Pan, W.; Li, K.; Jiang, Q.; Liu, G. Sliding trend fuzzy approximate entropy as a novel descriptor of heart rate variability in obstructive sleep apnea. IEEE J. Biomed. Health Inform. 2019, 23, 175–183. [Google Scholar] [CrossRef]
- Penzel, T.; Kantelhardt, J.W.; Grote, L.; Peter, J.H.; Bunde, A. Comparison of detrended fluctuation analysis and spectral analysis for heart rate variability in sleep and sleep apnea. IEEE Trans. Biomed. Eng. 2003, 50, 1143–1151. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhang, X.; Song, C. An automatic screening approach for obstructive sleep apnea diagnosis based on single-lead electrocardiogram. IEEE Trans. Autom. Sci. Eng. 2015, 12, 106–115. [Google Scholar] [CrossRef]
- Sapina, M.; Karmakar, C.K.; Kramaric, K.; Garcin, M.; Adelson, P.D.; Milas, K.; Piric, M.; Brdaric, D.; Yearwood, J. Multi-lag tone-entropy in neonatal stress. J. R. Soc. 2018, 15. [Google Scholar] [CrossRef]
- Ao, D.; Sun, R.; Tong, K.Y.; Song, R. Characterization of stroke- and aging-related changes in the complexity of EMG signals during tracking tasks. Ann. Biomed. Eng. 2015, 43, 990–1002. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, Y.H.; Lo, M.T.; Peng, C.K.; Huang, N.E.; Yang, C.C.; Kuo, T.B. Novel application of multi dynamic trend analysis as a sensitive tool for detecting the effects of aging and congestive heart failure on heart rate variability. Chaos Interdiscip. J. Nonlinear Sci. 2016, 26, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Gonzalez, S.; Navarro-Mesa, J.L.; Julia-Serda, G.; Kraemer, J.F.; Wessel, N.; Ravelo-Garcia, A.G. Heart rate variability feature selection in the presence of sleep apnea: An expert system for the characterization and detection of the disorder. Comput. Biol. Med. 2017, 91, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.L.P.D.; Rafael, P.; Luciano Neves, R.; Valter Luis, P.; Luciana Aparecida, C.; Niels, W.; Ovidiu Constantin, B. Heart rate detrended fluctuation indexes as estimate of obstructive sleep apnea severity. Medicine 2015, 94, e516. [Google Scholar] [CrossRef] [PubMed]
- Cysarz, D.; Bettermann, H.; Van, L.P. Entropies of short binary sequences in heart period dynamics. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2163. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cheng, J.; Yu, Y.; Luo, S. A rolling bearing fault diagnosis method based on multi-scale fuzzy entropy and variable predictive model-based class discrimination. Mech. Mach. Theory 2014, 78, 187–200. [Google Scholar] [CrossRef]
- Zheng, L.; Pan, W.; Li, Y.; Luo, D.; Wang, Q.; Liu, G. Use of mutual information and transfer entropy to assess interaction between parasympathetic and sympathetic activities of nervous system from HRV. Entropy 2017, 19, 489. [Google Scholar] [CrossRef] [Green Version]
- Vitelli, O.; Pozzo, M.D.; Baccari, G.; Rabasco, J.; Pietropaoli, N.; Barreto, M.; Villa, M.P. Autonomic imbalance during apneic episodes in pediatric obstructive sleep apnea. Clin. Neurophysiol. 2016, 127, 551–555. [Google Scholar] [CrossRef]
- Somers, V.K.; Dyken, M.E.; Clary, M.P.; Abboud, F.M. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Investig. 1995, 96, 1897–1904. [Google Scholar] [CrossRef] [Green Version]

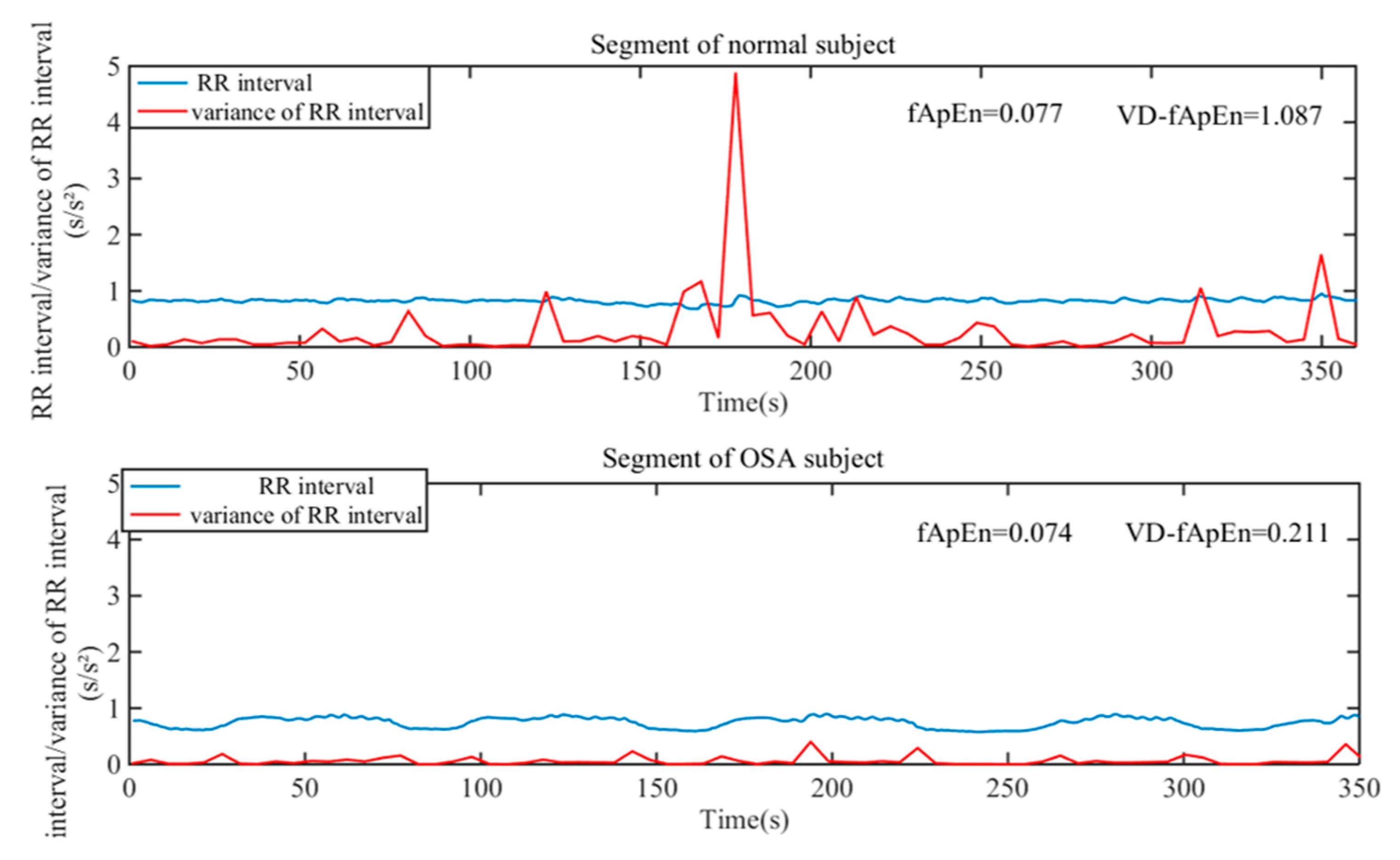
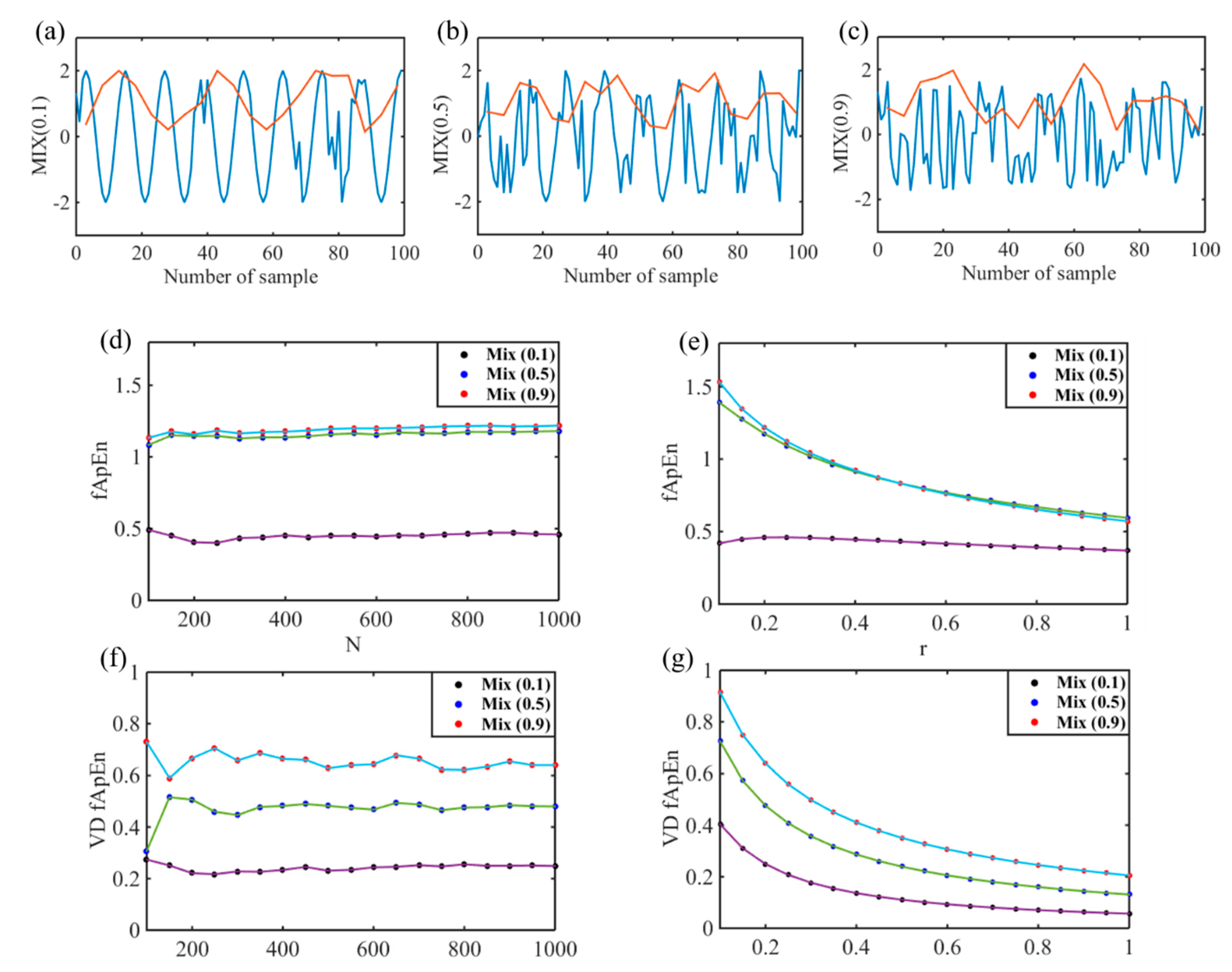

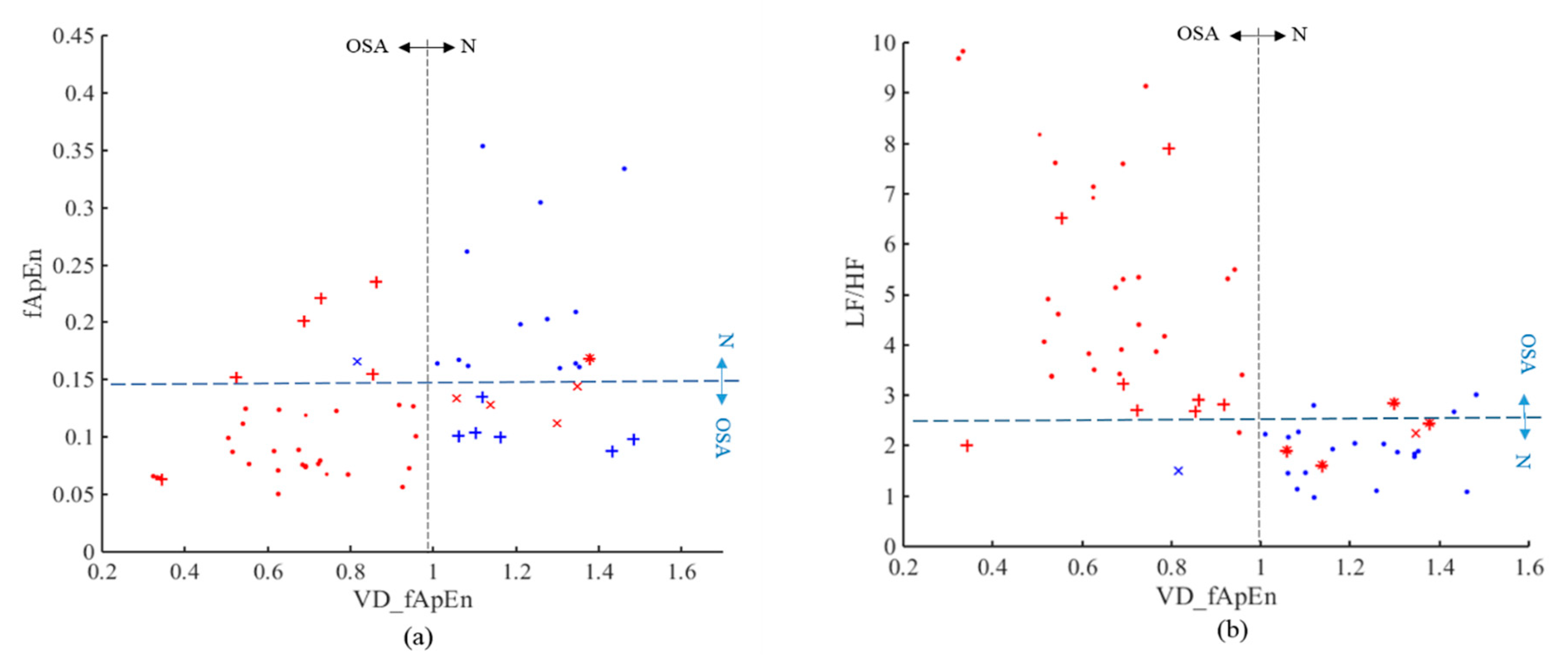
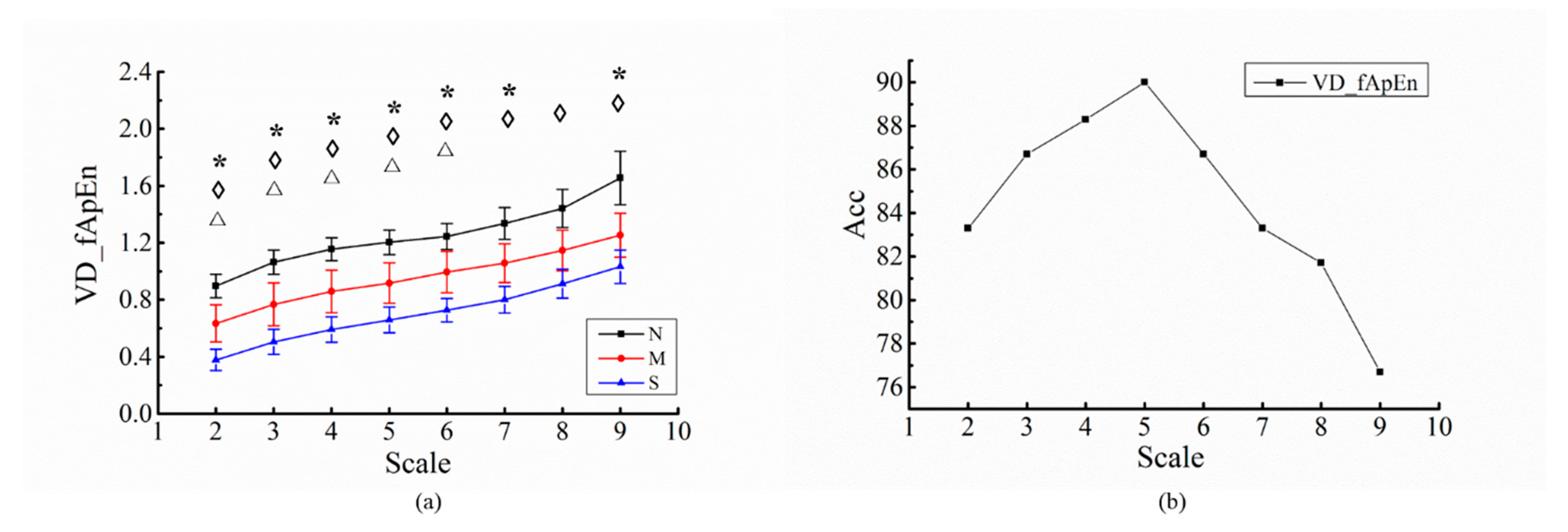
| Indices | N | M | S | p | |||
|---|---|---|---|---|---|---|---|
| N&M | N&S | M&S | |||||
| Frequency domain indices | LF | 0.004 ± 0.005 | 0.001 ± 0.0004 | 0.011 ± 0.025 | 0.638 | 0.190 | 0.097 |
| HF | 0.004 ± 0.005 | 0.0004 ± 0.0003 | 0.009 ± 0.028 | 0.642 | 0.315 | 0.166 | |
| LF/HF | 1.862 ± 0.576 | 4.325 ± 2.238 | 4.893 ± 2.279 | 0 *** | 0 *** | 0.367 | |
| Nonlinear indices | fApEn | 0.181 ± 0.077 | 0.100 ± 0.03 | 0.11 ± 0.054 | 0 *** | 0.001** | 0.407 |
| VD_fApEn | 1.204 ± 0.172 | 0.917 ± 0.283 | 0.659 ± 0.179 | 0 *** | 0 *** | 0 *** | |
| TP | TN | FP | FN | Accuracy | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|
| LF/HF | 28 | 20 | 0 | 12 | 80 | 70 | 100 |
| fApEn | 33 | 14 | 6 | 7 | 78.3 | 82.5 | 70 |
| VD_fApEn | 35 | 19 | 1 | 5 | 90 | 87.5 | 95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wu, S.; Yang, Q.; Liu, G.; Ge, L. Application of the Variance Delay Fuzzy Approximate Entropy for Autonomic Nervous System Fluctuation Analysis in Obstructive Sleep Apnea Patients. Entropy 2020, 22, 915. https://doi.org/10.3390/e22090915
Li Y, Wu S, Yang Q, Liu G, Ge L. Application of the Variance Delay Fuzzy Approximate Entropy for Autonomic Nervous System Fluctuation Analysis in Obstructive Sleep Apnea Patients. Entropy. 2020; 22(9):915. https://doi.org/10.3390/e22090915
Chicago/Turabian StyleLi, Yifan, Shan Wu, Quanan Yang, Guanzheng Liu, and Leijiao Ge. 2020. "Application of the Variance Delay Fuzzy Approximate Entropy for Autonomic Nervous System Fluctuation Analysis in Obstructive Sleep Apnea Patients" Entropy 22, no. 9: 915. https://doi.org/10.3390/e22090915






