Optimization Modeling of Irreversible Carnot Engine from the Perspective of Combining Finite Speed and Finite Time Analysis
Abstract
:1. Introduction
2. Optimization Models of Carnot Cycle Engine
2.1. Models in Thermodynamics in Finite Time Analysis Seeking for Maximum Power Output of Carnot Cycle Engine
2.2. Models of Irreversible Carnot Cycle Engine in Thermodynamics with Finite Speed
2.2.1. First Law of Thermodynamics for Processes with Finite Speed in Closed System
2.2.2. Model of Carnot Cycle Engine with Analytically Modeled Internal and External Irreversibility
- The irreversible heat received by the cycle gas from the source:
- The irreversible heat rejected by the cycle gas to the sink:
- The irreversible work produced/consumed during the isothermal processes of the cycle:
2.3. The Curzon–Ahlborn Model of the Carnot Cycle Engine Combined with the Analysis Based on Thermodynamics with Finite Speed (TFS)
- The absence of heat losses Qlost, in order to consider similar cycles in both analyses.
- The presence of losses in the work expression, so that the work lost in the two adiabatic processes due to finite speed is obtained by integrating the irreversible work for processes with finite speed in the processes 2-3′ and 4′-1 (Equation (2)) and subtracting the reversible work in the processes 2-3 and 4-1 (see Figure 1):
2.4. Unification Attempts of Thermodynamics in Finite Time and Thermodynamics with Finite Speed Analyses
- For w = 0, which means an internally reversible cycle, Equations (72) and (74) lead to = 1, so that Equation (75) becomes:
- For w ≠ 0, by combining Equations (74) and (76), a first approximation of the term responsible for cycle irreversibilities is expressed as:
- For internally reversible, externally irreversible Carnot cycle engine for which w = 0 and consequently, = 1, one gets the Curzon–Ahlborn “nice radical” [3]:
- For an internally and externally irreversible Carnot cycle engine for which w ≠ 0 and consequently, > 1, one gets:
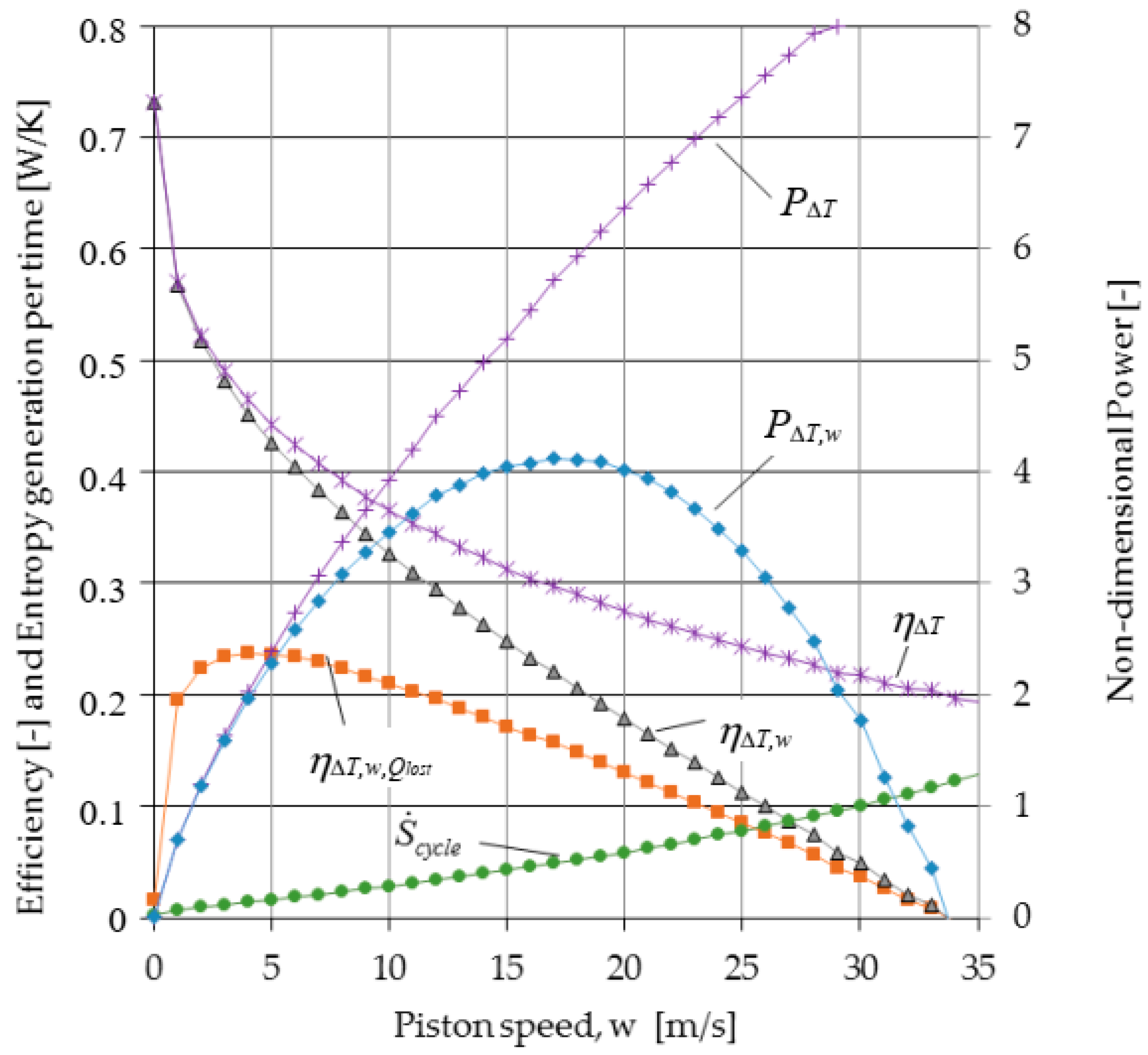
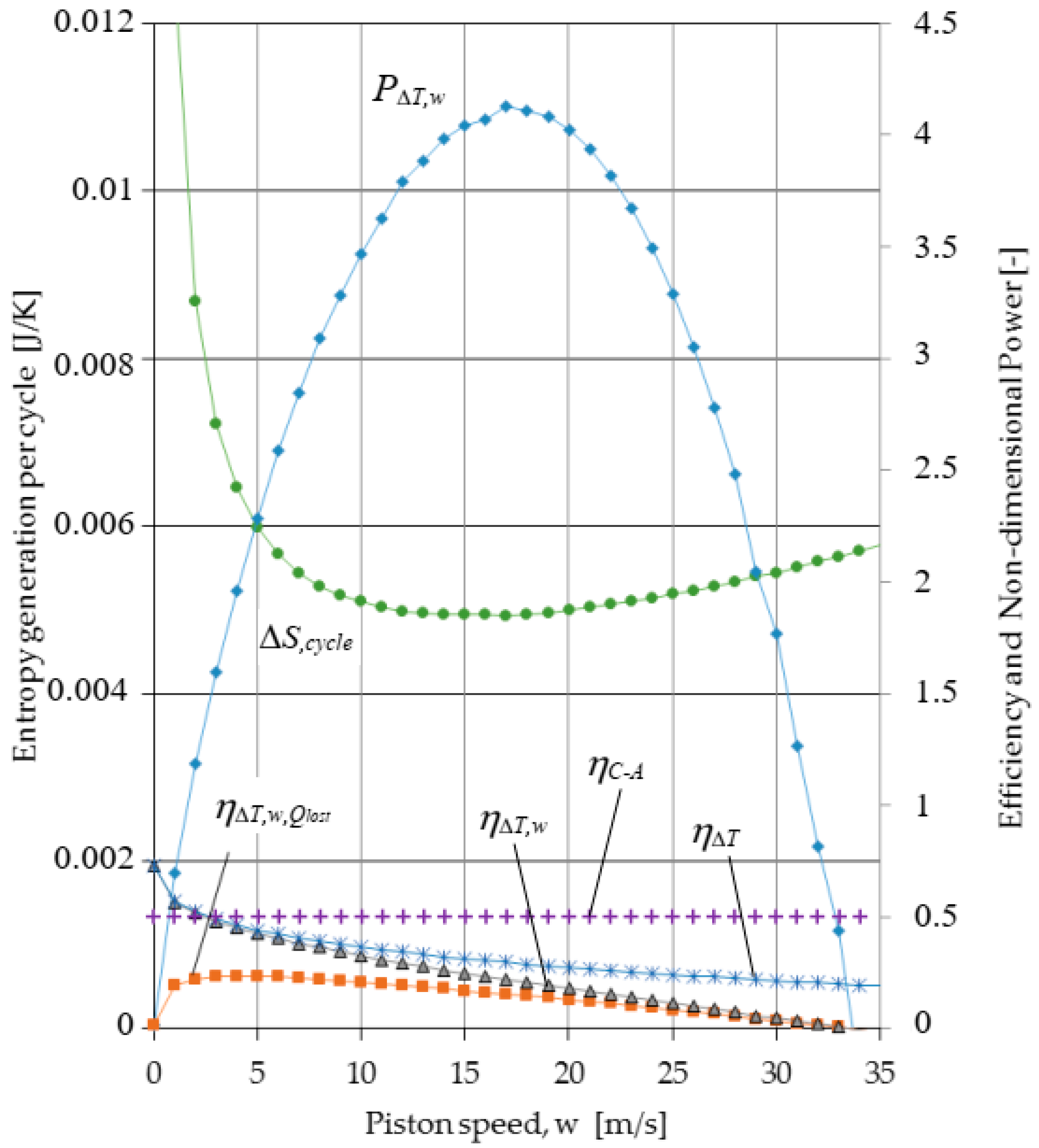
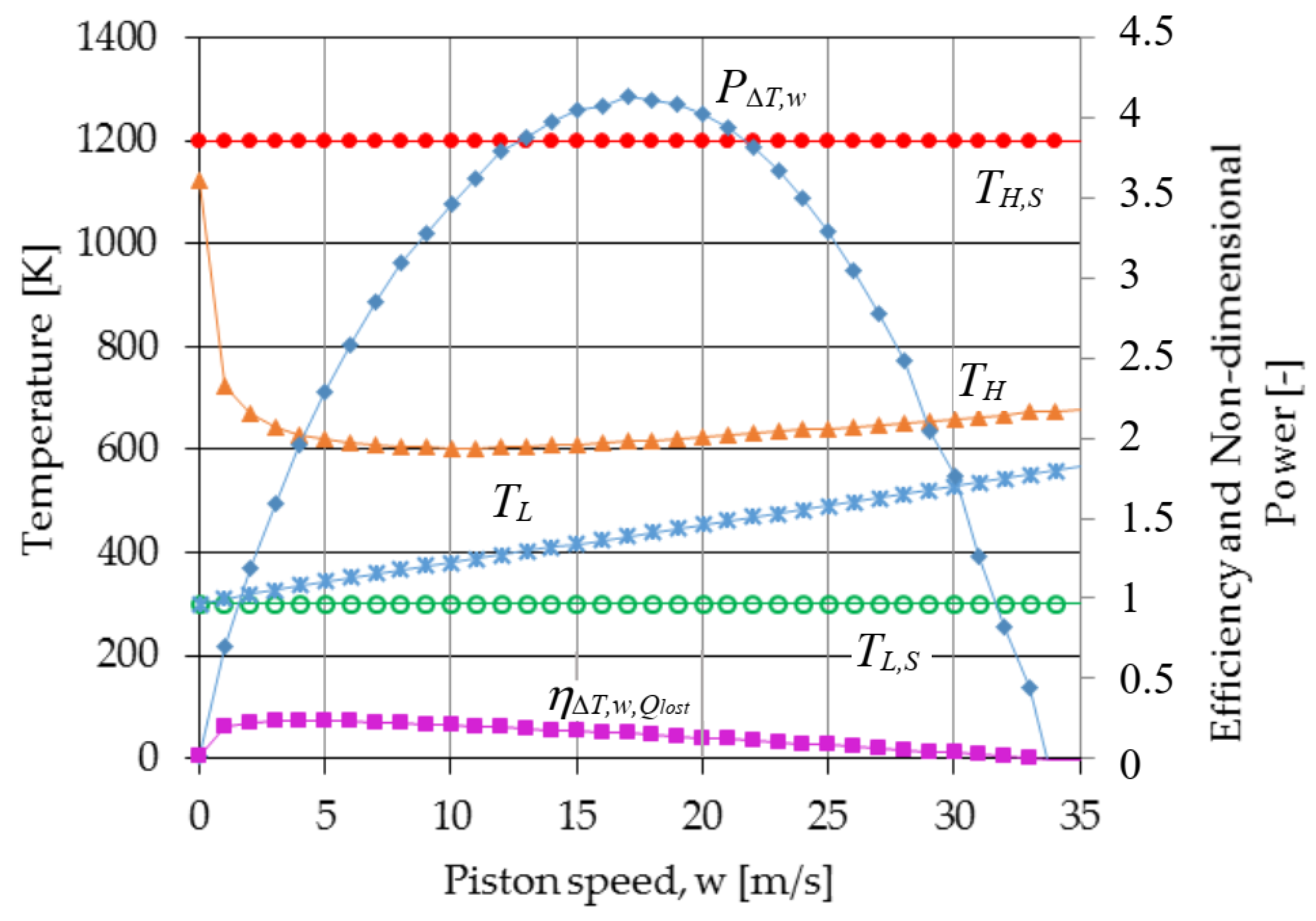
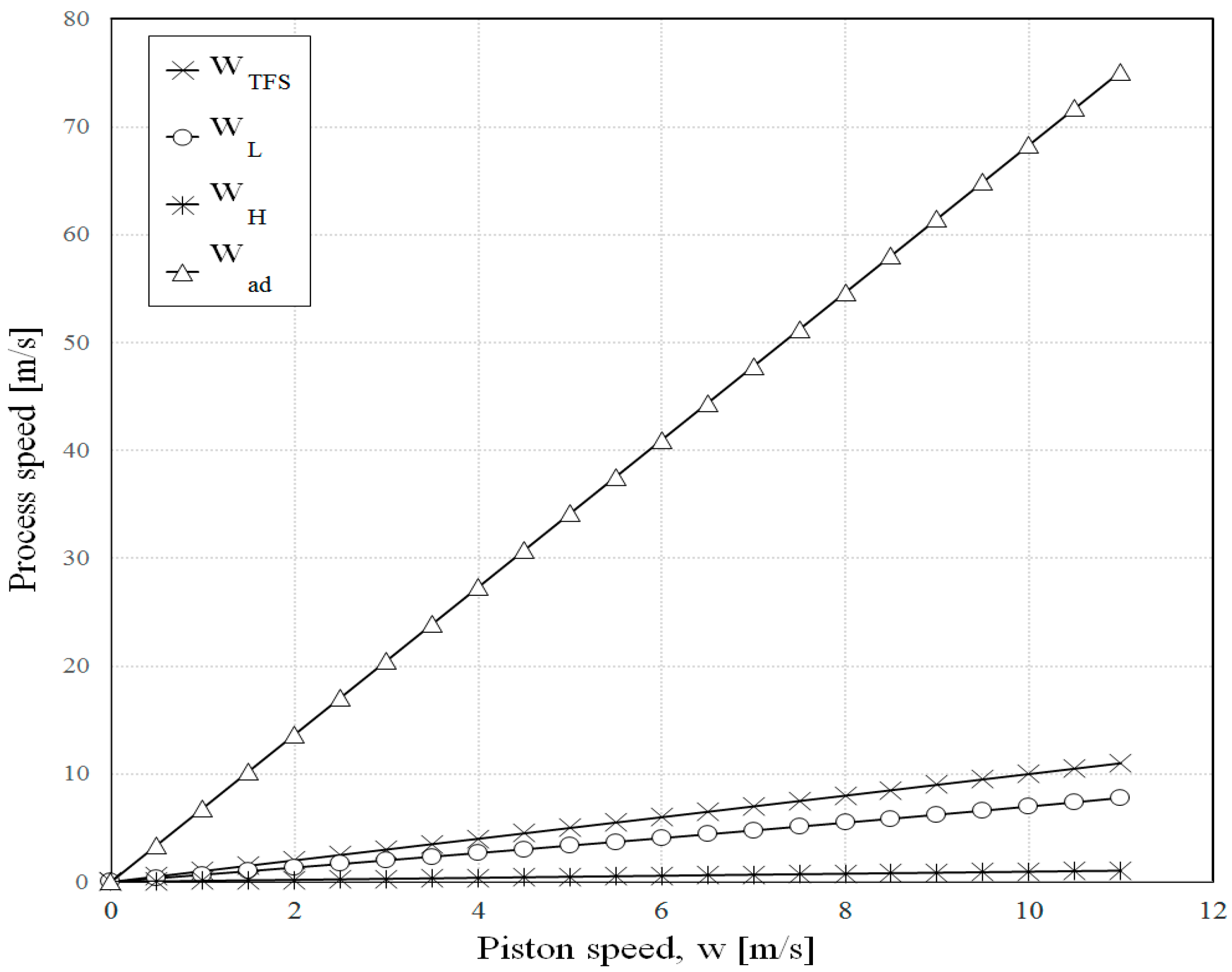
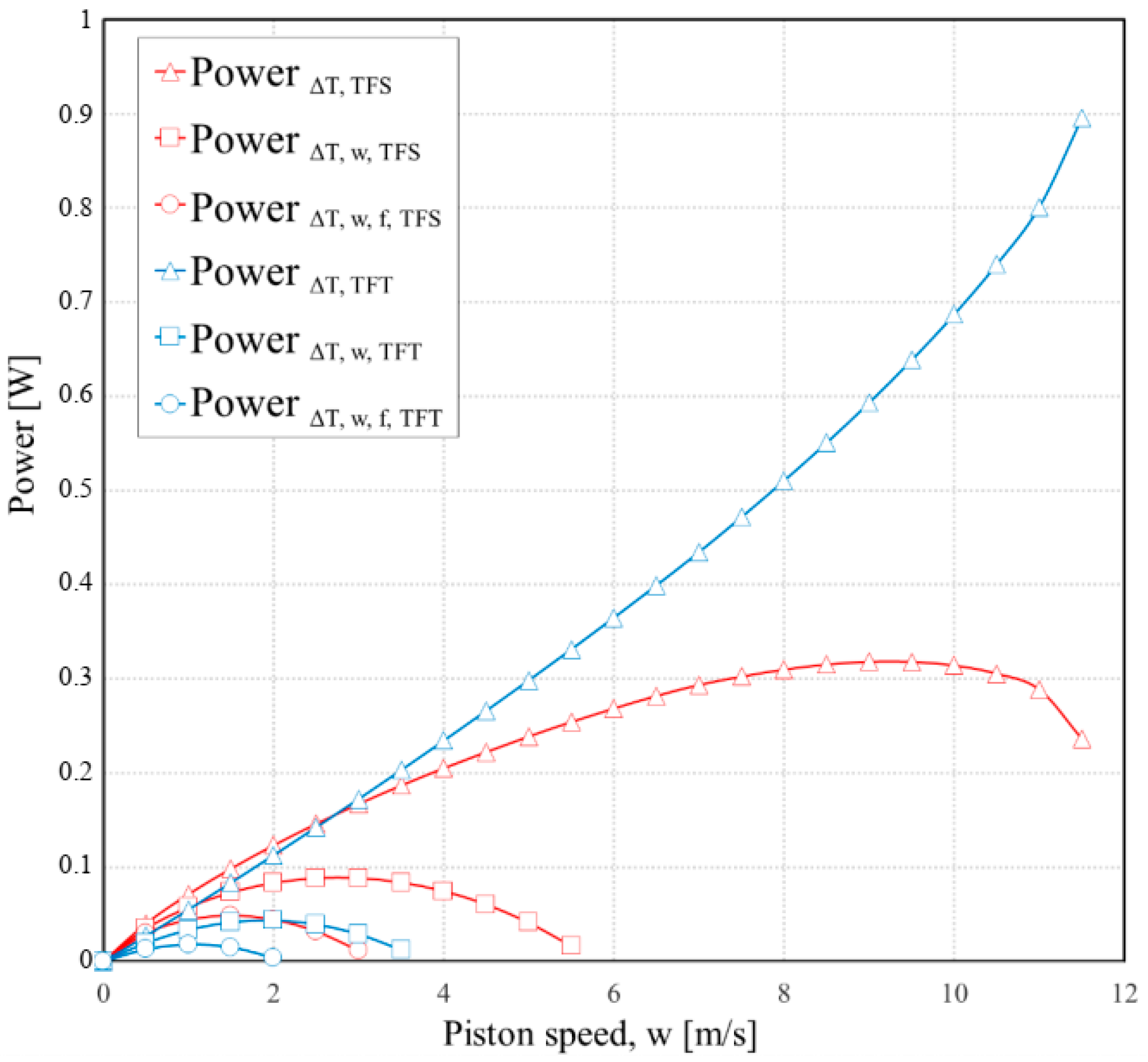
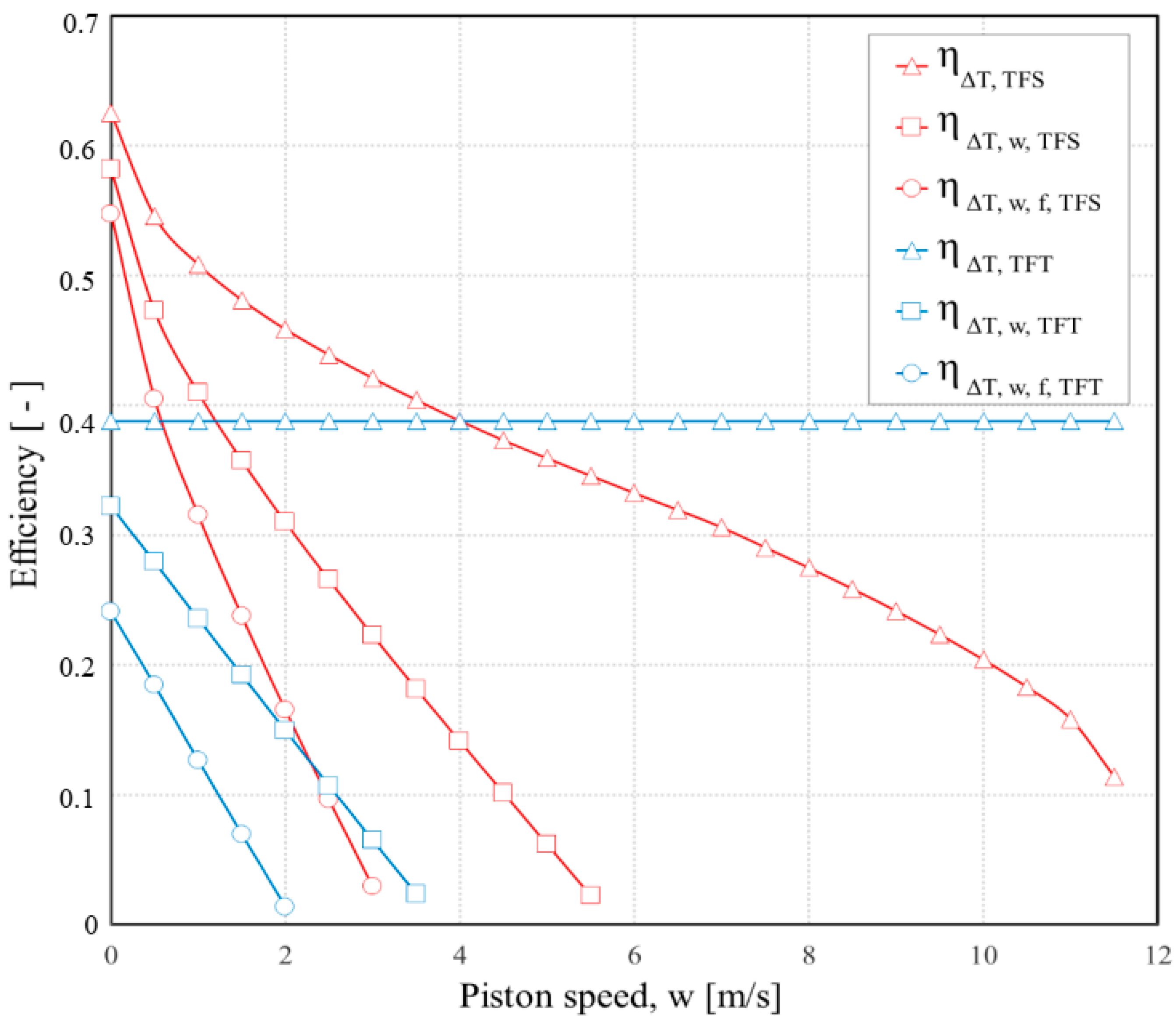
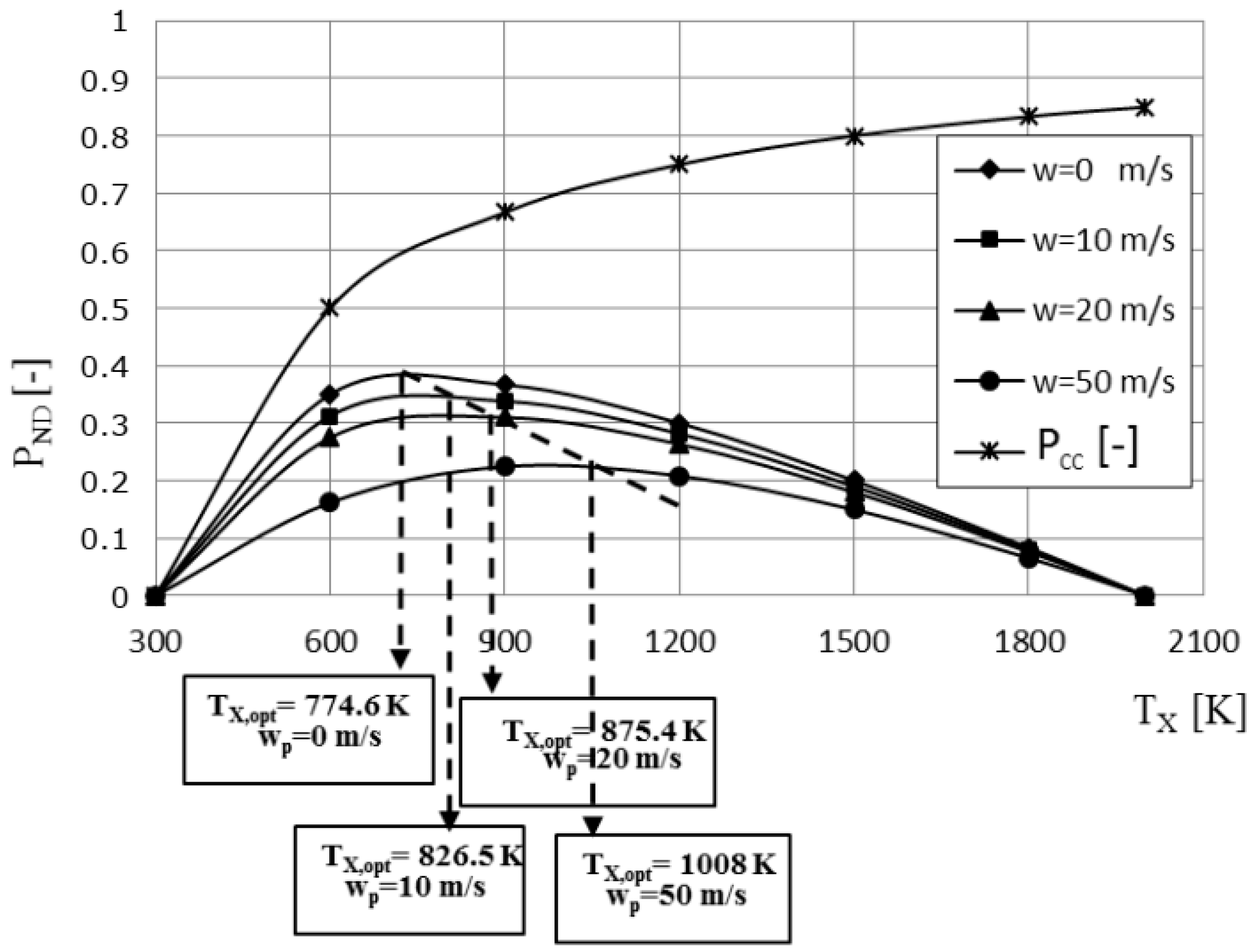
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salamon, P. Some Issues in Finite Time Thermodynamics. In Thermodynamic Optimization and Complex. Energy Systems; Bejan, A., Mamut, E., Eds.; NATO Science Series, 3. High Technology; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1999; Volume 69, pp. 421–424. [Google Scholar]
- Bejan, A. Advanced Engineering Thermodynamics; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Curzon, F.L.; Ahlborn, B. Efficiency of a carnot engine at maximum power output. Am. J. Phys. 1975, 43, 22–24. [Google Scholar] [CrossRef]
- Chambadal, P. Les Centrales Nucléaires; A. Colin: Paris, France, 1957. [Google Scholar]
- Novikov, I.I. The efficiency of atomic power stations (a review). In Journal of Nuclear Energy II; Pergamon Press Ltd.: London, UK, 1958; Volume 7, pp. 125–128. [Google Scholar]
- Gyftopulous, E.P. Fundamentals of analysis of processes. Energy Convers. Manag. 1997, 38, 1525–1533. [Google Scholar] [CrossRef]
- Moran, M.J. On second law analysis and the failed promises of finite time thermodynamics. Energy 1998, 23, 517–519. [Google Scholar] [CrossRef]
- Sekulic, D.P. A fallacious argument in the finite time thermodynamics concept of endoreversibilit. J. Appl. Phys. 1998, 83, 4561–4565. [Google Scholar] [CrossRef]
- Gyftopoulos, E.P. Infinite time (reversible) versus finite time (irreversible) thermodynamics: A misconceived distinction. Energy 1999, 24, 1035–1039. [Google Scholar] [CrossRef]
- Ishida, M. The role and limitations of endoreversible thermodynamics. Energy 1999, 24, 1009–1014. [Google Scholar] [CrossRef]
- Gyftopoulos, E.P. On the Curzon-Ahlborn efficiency and its lack of connection to power producing processes. Energy Convers. Manag. 2002, 43, 609–615. [Google Scholar] [CrossRef]
- Kodal, A.; Sahin, B.; Yilmaz, T.A. Comparative Performance analysis of irreversible Carnot engine under maximum power density and maximum power conditions. Energy Convers. Manag. 2000, 41, 235–248. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, L.; F Sun, F.; Wu, C. Optimal Performance of A Generalized Irreversible Carnot-Engine. Appl. Energy 2005, 81, 376–387. [Google Scholar] [CrossRef]
- Ladino-Luna, D. On optimization of a non-endoreversible curzon-ahlborn cycle. Entropy 2007, 9, 186–197. [Google Scholar] [CrossRef]
- Nie, W.; VHe, J.; Deng, X. Local stability analysis of an irreversible Carnot heat engine. Int. J. Therm. Sci. 2008, 47, 633–640. [Google Scholar] [CrossRef]
- Ibrahim, O.M.; Klein, S.A.; Mitchell, J.W. Economic Evaluation of the Maximum Power Efficiency Concept, ASME Winter Annual Meeting, Atlanta, Georgia, USA, 1–6 December 1991. J. Eng. Gas. Turbine Power 1991, 113, 514. [Google Scholar] [CrossRef]
- Wu, C.; Kiang, R. Finite-time thermodynamics analysis of a Carnot engine with irreversibility. Energy 1992, 17, 1173–1178. [Google Scholar] [CrossRef]
- Feidt, M. Optimal Thermodynamics—New Upperbounds. Entropy 2009, 11, 529–547. [Google Scholar] [CrossRef]
- Salamon, P. Physics versus engineering of finite-time thermodynamic models and optimizations. In Thermodynamic Optimization of Complex Energy Systems, NATO Advanced Study Institute, Neptun, Romania, 13–24 July 1998; Bejan, A., Mamut, E., Eds.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1999; pp. 421–424. [Google Scholar]
- Salamon, P. A contrast between the physical and the engineering approaches to finite-time thermodynamic models and optimizations. In Recent Advances in Finite Time Thermodynamics; Wu, C., Chen, L., Chen, J., Eds.; Nova Science Publishers: New York, NY, USA, 1999; pp. 541–552. [Google Scholar]
- Andresen, B. Comment on “A fallacious argument in the finite time thermodynamics concept of endoreversibility”. J. Appl. Phys. 2001, 90, 6557–6559. [Google Scholar] [CrossRef] [Green Version]
- Salamon, P.; Nulton, J.D.; Siragusa, G.; Andresen, T.R.; Limon, A. Principles of control thermodynamics. Energy 2001, 26, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.C.; Yan, Z.J.; Lin, G.X.; Andresen, B. On the Curzon-Ahlborn efficiency and its connection with the efficiencies of real heat engines. Energy Convers. Manag. 2001, 42, 173–181. [Google Scholar] [CrossRef]
- Tsirlin, A.; Sukin, I. Averaged optimization and finite-time thermodynamics. Entropy 2020, 22, 912. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Ayala, J.G.; Roco, J.M.M.; Medina, A.; Hernandez, A.C. optimization, stability, and entropy in endoreversible heat engines. Entropy 2020, 22, 1323. [Google Scholar] [CrossRef] [PubMed]
- Muschik, W.; Hoffmann, K.H. Modeling, simulation, and reconstruction of 2-reservoir heat-to-power processes in finite-time thermodynamics. Entropy 2020, 22, 997. [Google Scholar] [CrossRef] [PubMed]
- Insinga, A.R. The quantum friction and optimal finite-time performance of the quantum otto cycle. Entropy 2020, 22, 1060. [Google Scholar] [CrossRef]
- Masser, R.; Khodja, A.; Scheunert, M.; Schwalbe, K.; Fischer, A.; Paul, R.; Hoffmann, K.H. Optimized piston motion for an alpha-type stirling engine. Entropy 2020, 22, 700. [Google Scholar] [CrossRef]
- Abiuso, P.; Miller, H.J.D.; Perarnau-Llobet, M.; Scandi, M. Geometric optimization of quantum thermodynamic processes. Entropy 2020, 22, 1076. [Google Scholar] [CrossRef]
- Feng, H.J.; Qin, W.X.; Chen, L.G.; Cai, C.G.; Ge, Y.L.; Xia, S.J. Power output, thermal efficiency and exergy-based ecological performance optimizations of an irreversible KCS-34 coupled to variable temperature heat reservoirs. Energy Convers. Manag. 2020, 205, 112424. [Google Scholar] [CrossRef]
- Tang, C.Q.; Chen, L.G.; Feng, H.J.; Ge, Y.L. Four-objective optimization for an irreversible closed modified simple Brayton cycle. Entropy 2021, 23, 282. [Google Scholar] [CrossRef]
- Chen, L.; Feng, H.; Ge, Y. Power and efficiency optimization for open combined regenerative brayton and inverse brayton cycles with regeneration before the inverse cycle. Entropy 2020, 22, 677. [Google Scholar] [CrossRef]
- Andresen, B. Finite-Time Thermodynamics; Physics Laboratory II; University of Copenhagen: Copenhagen, Denmark, 1983. [Google Scholar]
- Şahin, B.; Kodal, A.; Yavuz, H. Maximum power density foe an endoreversible Carnot heat engine. Energy 1996, 21, 1219–1225. [Google Scholar] [CrossRef]
- Agnew, B.; Anderson, A.; Frost, T.H. Optimization of a steady-flow Carnot cycle with external irreversibilities for maximum specific output. Appl. Therm. Eng. 1997, 17, 3–15. [Google Scholar] [CrossRef]
- Bojic, M. Cogeneration of power and heat by using endoreversible Carnot engine. Energy Convers. Manag. 1997, 38, 1877–1880. [Google Scholar] [CrossRef]
- Qin, X.; Chen, L.; Sun, F.; Wu, C. Frequency-dependent performance of an endoreversible Carnot engine with a linear phenomenological heat-transfer. Appl. Energy 2005, 81, 365–375. [Google Scholar]
- Ding, Z.; Chen, L.; Sun, F. Finite time exergoeconomic performance for six endoreversible heat engine cycles: Unified description. Appl. Math. Model. 2011, 35, 728–736. [Google Scholar] [CrossRef]
- Tierney, M. Minimum exergy destruction from endoreversible and finite-time thermodynamics machines and their concomitant indirect energy. Energy 2020, 197, 117184. [Google Scholar] [CrossRef]
- Feidt, M. Thermodynamique et Optimisation Energetique des Systèmes et Procédés, 2nd ed.; Technique et Documentation; Lavoisier: Paris, France, 1996. [Google Scholar]
- Chen, J. The maximum power output and maximum efficiency of an irreversible carnot heat engine. J. Phys. D Appl. Phys. 1994, 27, 1144–1149. [Google Scholar] [CrossRef]
- Chen, L.; Sun, F.; Wu, C. A generalized model of real heat engines and its performance. J. Inst. Energy 1996, 69, 214–222. [Google Scholar]
- Feidt, M.; Costea, M.; Petrescu, S.; Stanciu, C. Nonlinear thermodynamic analysis and optimization of a carnot engine cycle. Entropy 2016, 18, 243. [Google Scholar] [CrossRef] [Green Version]
- Feidt, M. Thermodynamics of energy systems and processes: A review and perspectives. J. Appl. Fluid Mech. 2012, 5, 85–98. [Google Scholar]
- Feidt, M. Thermodynamique Optimale en Dimensions Physiques Finies; Lavoisier: Paris, France, 2013. [Google Scholar]
- Petrescu, S.; Stanescu, G.; Costea, M. The study for optimization of the Carnot cycle which develops with finite speed. In Proceedings of the International Conference on Energy Systems and Ecology, ENSEC’93, Cracow, Poland, 5–8 July 1993; Volume 1, pp. 269–277. [Google Scholar]
- Petrescu, S.; Harman, C.; Bejan, A. The Carnot cycle with external and internal irreversibilities. In Proceedings of the Florence World Energy Research Symposium, Energy for the 21st Century: Conversion, Utilization and Environmental Quality, Firenze, Italy, 6–8 July 1994. [Google Scholar]
- Petrescu, S.; Feidt, M.; Harman, C.; Costea, M. Optimization of the irreversible Carnot cycle engine for maximum efficiency and maximum power through use of finite speed thermodynamic analysis. In Proceedings of the ECOS’2002 Conference, Berlin, Germany, 3–5 July 2002; Tsatsaronis, G., Moran, M., Cziesla, F., Bruckner, T., Eds.; Volume 2, pp. 1361–1368. [Google Scholar]
- Petrescu, S.; Harman, C.; Costea, M.; Feidt, M. Thermodynamics with Finite Speed versus Thermodynamics in Finite Time in the Optimization of Carnot Cycle. In Proceedings of the 6-th ASME-JSME Thermal Engineering Joint Conference, Hawaii, HI, USA, 16–20 March 2003. [Google Scholar]
- Petre, C. Utilizarea Termodinamicii cu Viteza Finita in Studiul si Optimizarea Ciclului Carnot si a Masinilor Stirling (The Use of Thermodynamics with Finite Speed to the Study and Optimization of Carnot Cycle and Stirling Machines). Ph.D. Thesis, University Politehnica of Bucharest, Bucharest, Romania, University H. Poincaré of Nancy, Nancy, France, 2007. [Google Scholar]
- Feidt, M.; Costea, M.; Petre, C.; Petrescu, S. Optimization of direct Carnot cycle. Appl. Therm. Eng. 2007, 27, 829–839. [Google Scholar] [CrossRef]
- Petrescu, S.; Harman, C.; Bejan, A.; Costea, M.; Dobre, C. Carnot cycle with external and internal irreversibilities analyzed in thermodynamics with finite speed with the direct method. Termotehnica 2011, 15, 7–17. [Google Scholar]
- Liu, X.W.; Chen, L.G.; Wu, F.; Sun, F.R. Optimal performance of a spin quantum Carnot heat engine with multi-irreversibilities. J. Energy Inst. 2014, 87, 69–80. [Google Scholar] [CrossRef]
- Feng, H.J.; Chen, L.G.; Sun, F.R. Optimal Ratios of the Piston Speeds for a Finite Speed Endoreversible Carnot Heat Engine Cycle. Rev. Mex. Fis. 2010, 56, 135–140. [Google Scholar]
- Feng, H.J.; Chen, L.G.; Sun, F.R. Optimal ratio of the piston for a finite speed irreversible carnot heat engine cycle. Int. J. Sustain. Energy 2010, 30, 321–335. [Google Scholar] [CrossRef]
- Feng, H.J.; Chen, L.G.; Sun, F.R. Effects of unequal finite speed on the optimal performance of endoreversible Carnot refrigeration and heat pump cycles. Int. J. Sustain. Energy 2011, 30, 289–301. [Google Scholar] [CrossRef]
- Yang, B.; Chen, L.G.; Sun, F.R. Performance analysis and optimization for an endoreversible Carnot heat pump cycle with finite speed of piston. Int. J. Energy Environ. 2011, 2, 1133–1140. [Google Scholar]
- Chen, L.G.; Feng, H.J.; Sun, F.R. Optimal piston speed ratios for irreversible carnot refrigerator and heat pump using finite time thermodynamics, finite speed thermodynamics and the direct method. J. Energy Inst. 2011, 84, 105–112. [Google Scholar] [CrossRef]
- Petrescu, S.; Harman, C.; Costea, M.; Florea, T.; Petre, C. Advanced Energy Conversion; Bucknell University: Lewisburg, PA, USA, 2006. [Google Scholar]
- Petrescu, S.; Costea, M.; Petrescu, V.; Malancioiu, O.; Boriaru, N.; Stanciu, C.; Banches, E.; Dobre, C.; Maris, V.; Leontiev, C. Development of Thermodynamics with Finite Speed and Direct Method; AGIR: Bucharest, Romania, 2011. [Google Scholar]
- Petrescu, S.; Costea, M.; Feidt, M.; Ganea, I.; Boriaru, N. Advanced Thermodynamics of Irreversible Processes with Finite Speed and Finite Dimensions. A Historical and Epistemological Approach, with Extension to Biological and Social Systems; AGIR: Bucharest, Romania, 2015. [Google Scholar]
- Costea, M.; Petrescu, S.; Harman, C. The effect of irreversibilities on solar stirling engine cycle performance. Energy Convers. Manag. 1999, 40, 1723–1731. [Google Scholar] [CrossRef]
- Petrescu, S.; Costea, M.; Harman, C.; Florea, T. Application of the direct method to irreversible stirling cycles with finite speed. Int. J. Energy Res. 2002, 26, 589–609. [Google Scholar] [CrossRef]
- Florea, T.; Petrescu, S.; Florea, E. Schemes for Computation and Optimization of the Irreversible Processes in Stirling Machines; Leda & Muntenia: Constanta, Romania, 2000. [Google Scholar]
- Petrescu, S.; Cristea, A.F.; Boriaru, N.; Costea, M.; Petre, C. Optimization of the Irreversible Otto Cycle using Finite Speed Thermodynamics and the Direct Method. In Proceedings of the 10th WSEAS Int. Conf. on Mathematical and Computational Methods in Science and Engineering (MACMESE’08), Computers and Simulation in Modern Science, Bucharest, Romania, 7–9 November 2008; Mastorakis, N., Ed.; World Scientific and Engineering Academy and Society (WSEAS): Stevens Point, WI, USA; Volume 2, pp. 51–56. [Google Scholar]
- Cullen, B.; McGovern, J.; Petrescu, S.; Feidt, M. Preliminary modelling results for otto-stirling hybrid. cycle. In Proceedings of the ECOS 2009, Foz de Iguasu, Parana, Brazil, 31 August–3 September 2009; pp. 2091–2100. [Google Scholar]
- McGovern, J.; Cullen, B.; Feidt, M.; Petrescu, S. Validation of a simulation model for a combined otto and stirling cycle power plant. In Proceedings of the ASME 2010, 4th International Conference on Energy Sustainability, ES2010, Phoenix, AZ, USA, 17–22 May 2010. [Google Scholar]
- Petrescu, S.; Boriaru, N.; Costea, M.; Petre, C.; Stefan, A.; Irimia, C. Optimization of the irreversible diesel cycle using finite speed thermodynamics and the direct method. Bull. Transilv. Univ. Braşov 2009, 2, 87–94. [Google Scholar]
- Petrescu, S.; Petrescu, V.; Stanescu, G.; Costea, M. A Comparison between Optimization of Thermal Machines and Fuel Cells based on New Expression of the First Law of Thermodynamics for Processes with Finite Speed. In Proceedings of the 1st Conference on Energy ITEC’ 93, Marrakesh, Morocco, 6–10 June 1993; pp. 654–657. [Google Scholar]
- Petrescu, S. Contributions to the Study of Interactions and Processes of Non-Equilibrium in Thermal Machine. Ph.D. Thesis, Polytechnic Institute of Bucharest, Bucharest, Romania, 1969. [Google Scholar]
- Stoicescu, L.; Petrescu, S. The First Law of Thermodynamics for Processes with Finite Constant Speed in Closed Systems. Polytech. Inst. Buchar. Bull. 1964, 26, 87–108. [Google Scholar]
- Stoicescu, L.; Petrescu, S. Thermodynamic processes developing with constant finite speed. Polytech. Inst. Buchar. Bull. 1964, 26, 79–119. [Google Scholar]
- Stoicescu, L.; Petrescu, S. Thermodynamic processes developing with variable finite speed. Polytech. Inst. Buchar. Bull. 1965, 27, 65–96. [Google Scholar]
- Stoicescu, L.; Petrescu, S. Thermodynamic cycles with finite speed. Polytech. Inst. Buchar. Bull. 1965, 27, 82–95. [Google Scholar]
- Petrescu, S. Lectures on New Sources of Energy; Helsinki University of Technology: Otaniemi, Finland, 1991. [Google Scholar]
- Petrescu, S.; Iordache, R.; Stanescu, G.; Dobrovicescu, A. The First Law of Thermodynamics for Closed Systems, Considering the Irreversibilities Generated by Friction Piston-Cylinder, the Throttling of the Working Medium and the Finite Speed of Mechanical Interaction. In Proceedings of the ECOS’92, Zaragoza, Spain, 15–19 June 1992. [Google Scholar]
- Petrescu, S.; Stanescu, G. The direct method for studying the irreversible processes undergoing with finite speed in closed systems. Termotehnica 1993, 1, 69–84. [Google Scholar]
- Petrescu, S.; Harman, C. The Connection between the First and Second Law of Thermodynamics for Processes with Finite Speed. A Direct Method for Approaching and Optimization of Irreversible Processes. J. Heat Transf. Soc. Jpn. 1994, 33, 60–67. [Google Scholar]
- Stanescu, G. The Study of the Mechanism of Irreversibility Generation in Order to Improve the Performances of Thermal Machines and Devices. Ph.D. Thesis, U.P.B., Bucharest, Romania, 1993. [Google Scholar]
- Petrescu, S.; Petre, C.; Costea, M.; Malancioiu, O.; Boriaru, N.; Dobrovicescu, A.; Feidt, M.; Harman, C. A methodology of computation, design and optimization of solar stirling power plant using hydrogen/oxygen fuel cells. Energy 2010, 35, 729–739. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw-Hill: New York, NY, USA, 1988. [Google Scholar]
- Hagen, K.D. Heat Transfer with Applications; Prentice Hall Inc.: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Costea, M. Improvement of Heat Exchangers Performance in View of the Thermodynamic Optimization of Stirling Machine; Unsteady-State Heat Transfer in Porous Media. Ph.D. Thesis, University Politehnica of Bucharest, Bucharest, Romania, University H. Poincaré of Nancy, Nancy, France, 1997. [Google Scholar]
- Hosseinzade, H.; Sayyaadi, H.; Babaelahi, M. A new closed-form analytical thermal model for simulating Stirling engines based on polytropic—Finite speed thermodynamics. Energy Convers. Manag. 2015, 90, 395–408. [Google Scholar] [CrossRef]
- Hosseinzade, H.; Sayyaadi, H. CAFS: The combined adiabatic–finite speed thermal model for simulation and optimization of stirling engines. Energy Convers. Manag. 2015, 91, 32–53. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Ahmadi, M.A.; Pourfayaz, F.; Bidi, M.; Hosseinzade, H.; Feidt, M. Optimization of powered stirling heat engine with finite speed thermodynamics. Energy Convers. Manag. 2016, 108, 96–105. [Google Scholar] [CrossRef]
- Wu, H.; Ge, Y.L.; Chen, L.G.; Feng, H.J. Power, efficiency, ecological function and ecological coefficient of performance optimizations of irreversible Diesel cycle based on finite piston speed. Energy 2021, 216, 119235. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costea, M.; Petrescu, S.; Feidt, M.; Dobre, C.; Borcila, B. Optimization Modeling of Irreversible Carnot Engine from the Perspective of Combining Finite Speed and Finite Time Analysis. Entropy 2021, 23, 504. https://doi.org/10.3390/e23050504
Costea M, Petrescu S, Feidt M, Dobre C, Borcila B. Optimization Modeling of Irreversible Carnot Engine from the Perspective of Combining Finite Speed and Finite Time Analysis. Entropy. 2021; 23(5):504. https://doi.org/10.3390/e23050504
Chicago/Turabian StyleCostea, Monica, Stoian Petrescu, Michel Feidt, Catalina Dobre, and Bogdan Borcila. 2021. "Optimization Modeling of Irreversible Carnot Engine from the Perspective of Combining Finite Speed and Finite Time Analysis" Entropy 23, no. 5: 504. https://doi.org/10.3390/e23050504









