0D Dynamic Modeling and Experimental Characterization of a Biomass Boiler with Mass and Energy Balance
Abstract
:1. Introduction
2. Description of the Biomass Boiler
2.1. Experimental Setup
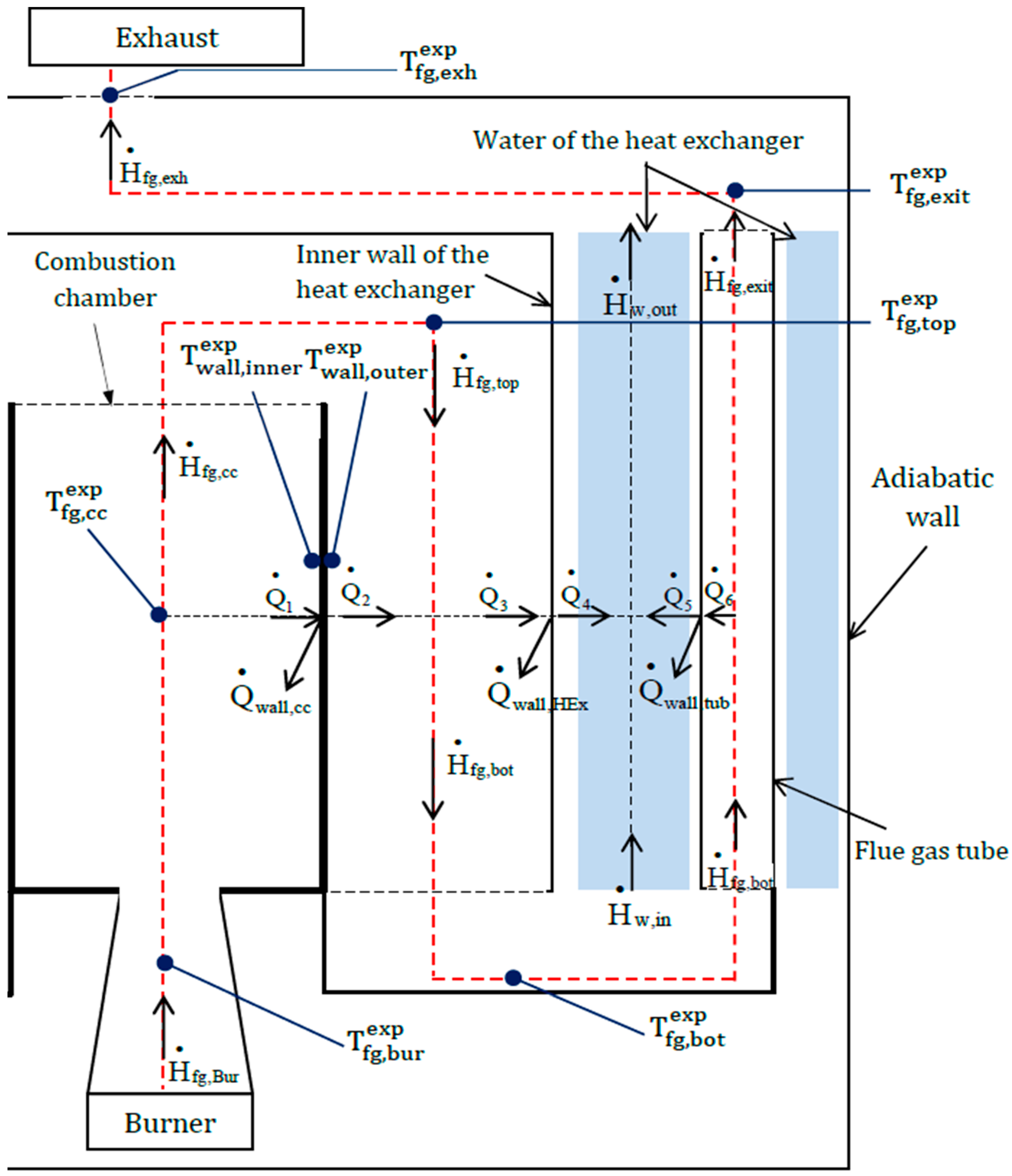
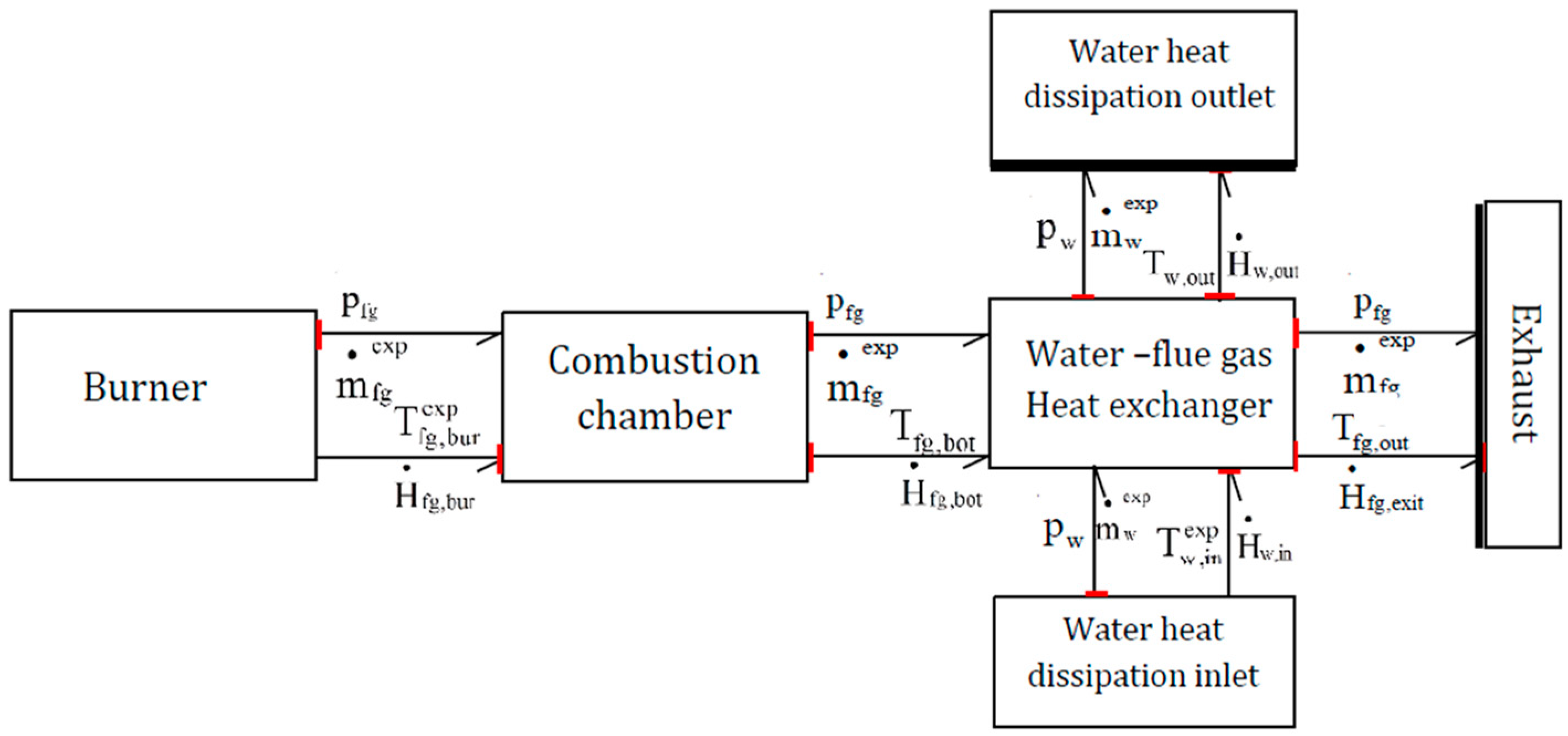
2.2. Energy Balance of the Boiler
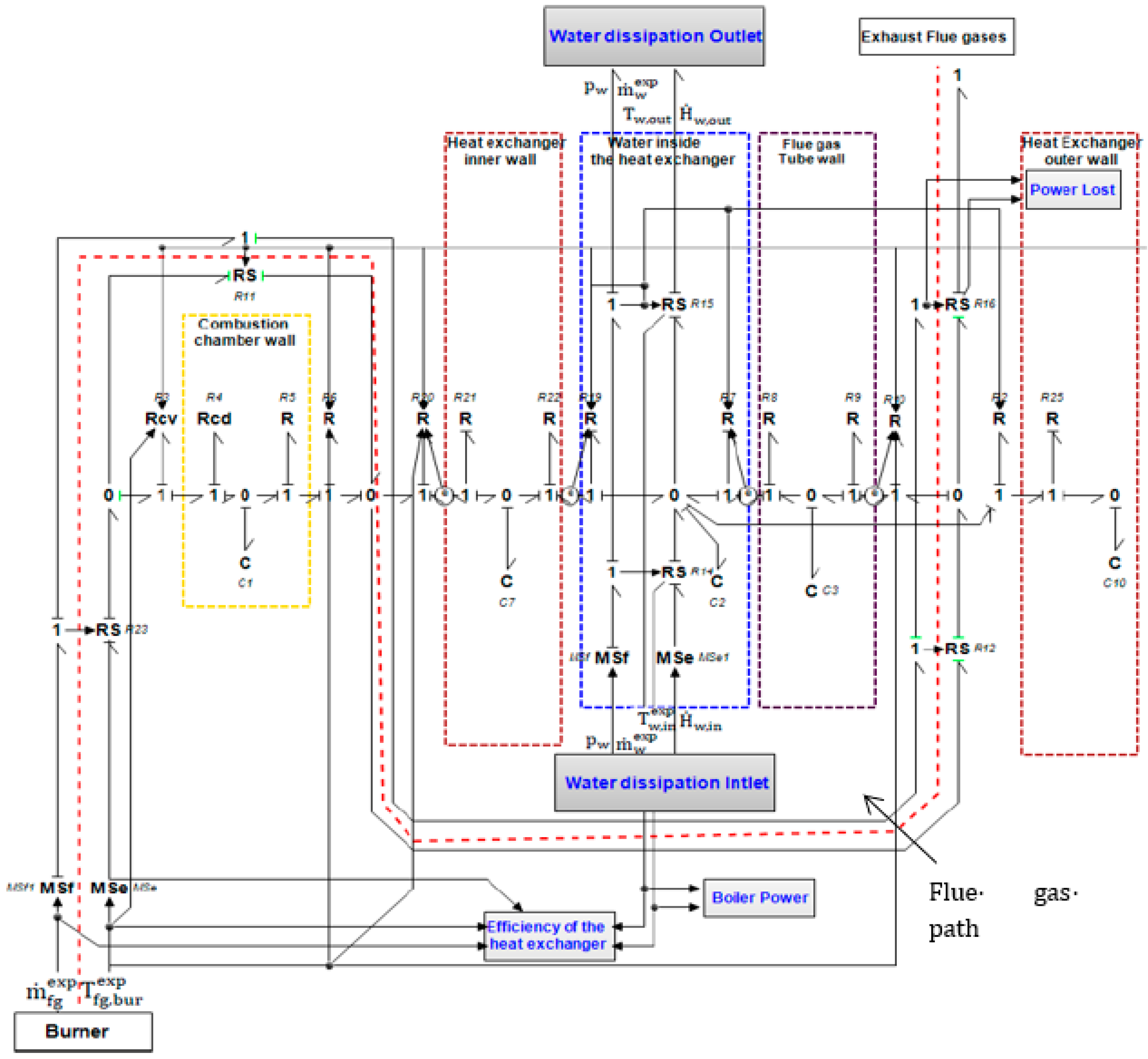
3. 0D Bond Graph Modeling
3.1. 0D Model of the Boiler

- -
- hg = 300 W.m−2.K−1 for t = 0–170 min
- -
- hg = 100 W.m−2.K−1 for t = 170–250 min.
3.2. Flue Gas Thermodynamic Properties
3.3. Solver Scheme
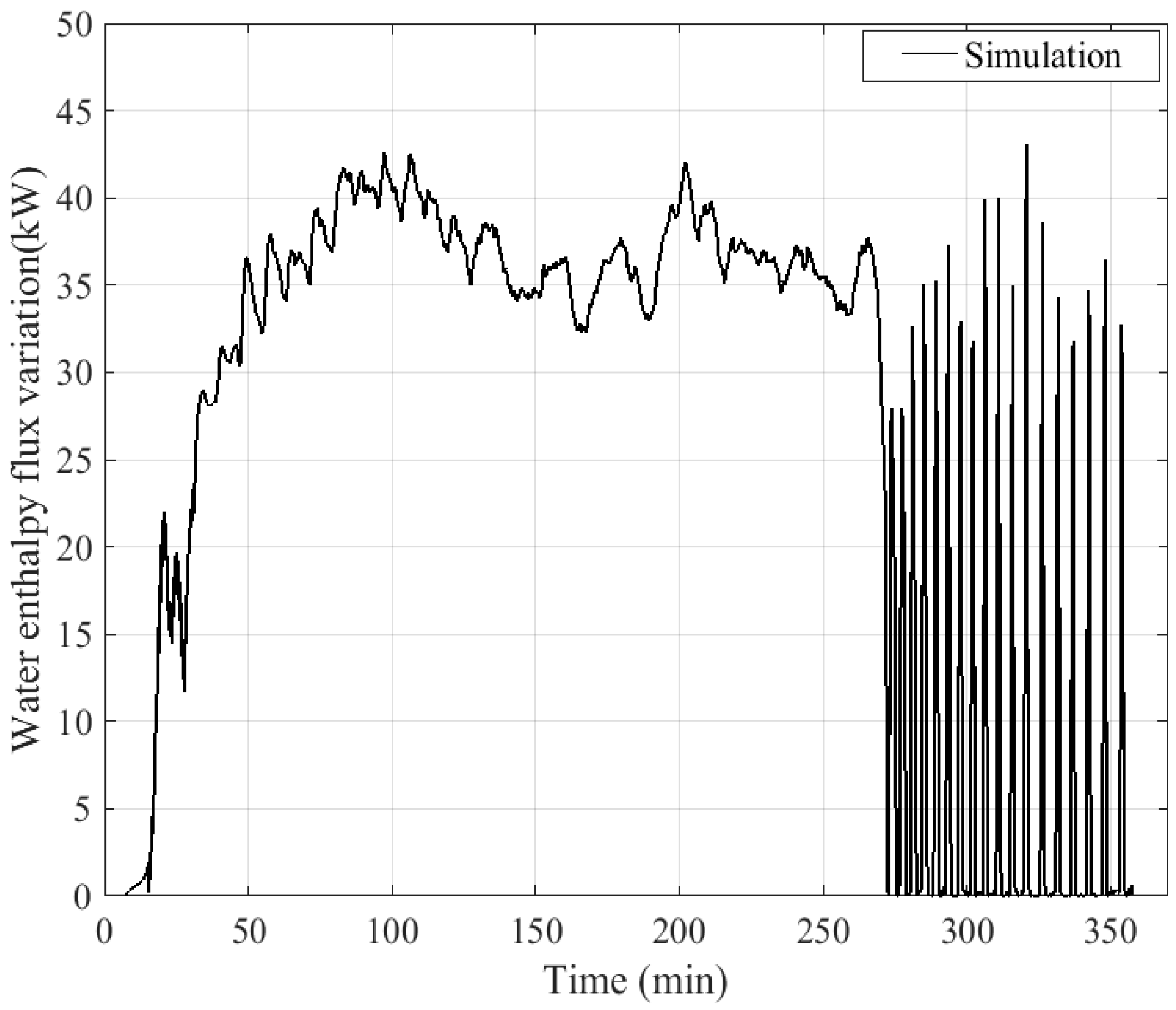
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| cp,fg(T) | Flue gas specific heat (J.kg−1.K−1) at constant pressure, function of temperature T |
| cw | Water specific heat (J.kg−1.K−1) |
| cwall | Wall specific heat (J.kg−1.K−1) |
| Dh | Hydraulic diameter (m) |
| H | Combustion chamber height (m) |
| hg | Global thermal transfer coefficient (W.m−2.K−1) |
| Enthalpy flux of the flue gas in a specific area (W) | |
| Enthalpy flux of the water in a specific area (W) | |
| k1 | First slope of Runge-Kutta fourth order formula |
| k2 | Second slope of Runge-Kutta fourth order formula |
| k3 | Third slope of Runge-Kutta fourth order formula |
| k4 | Fourth slope of Runge-Kutta fourth order formula |
| mwall | Wall mass (kg) |
| Experimental flue gas mass flow rate (kg.s−1) | |
| Experimental pellets mass flow rate (kg.s−1)) | |
| Experimental water mass flow rate (kg.s−1) | |
| pw | Water pressure (Pa) |
| pfg | Flue gas pressure (Pa) |
| Heat flux transferred to the water (W) | |
| Heat flux stored in the boiler structure (W) | |
| Convective heat flux exchanged between the flue gas and the combustion chamber wall (W) | |
| Heat flux stored in the heat exchanger wall (W) | |
| Heat flux stored in the walls of the flue gas tubes (W) | |
| Inside radius (m) | |
| Outside radius (m) | |
| Rcd | Conduction resistance (K.W−1) |
| Rcv | Convective resistance (K.W−1) |
| S | Exchange surface (m2) |
| t | Time (s) |
| Tamb | Ambient temperature (K) |
| Experimental flue gas temperature at the bottom of the heat exchanger (K) | |
| Experimental flue gas temperature in the burner (K) | |
| Experimental flue gas temperature in the combustion chamber (K) | |
| Experimental flue gas temperature in the chimney (boiler exhaust) (K) | |
| Experimental flue gas temperature at the flue gas tubes outlet (K) | |
| Experimental water temperature at the inlet of the heat exchanger (K) | |
| Experimental water temperature at the outlet of the heat exchanger (K) | |
| Calculated flue gas temperature at the RS-element inlet (K) | |
| Calculated flue gas temperature at the RS-element outlet (K) | |
| Experimental flue gas temperature at the top of the combustion chamber (K) | |
| Experimental temperature of the inner wall of the combustion chamber (K) | |
| Experimental temperature of the outer wall of the combustion chamber (K) | |
| Twall | Calculated wall temperature (K) |
| Subscripts | |
| air | Air |
| amb | Ambient |
| bur | Burner |
| bot | Bottom |
| cc | Combustion chamber |
| cd | Conductive |
| cv | Convective |
| exit | Exit |
| exh | Exhaust |
| fg | Flue gas |
| g | Global |
| HEx | Heat Exchanger |
| in | Inlet |
| rad | Radiative |
| tub | Tube |
| top | Top |
| tot | Total |
| out | Outlet |
| w | Water |
| wall | Wall |
| Superscript | |
| exp | Experimental Value |
| Greek symbols | |
| Variation of thermodynamic quantity | |
| λi | Wall thermal conductivity (W.m−1.K−1) |
| λfg | Flue gas thermal conductivity (W.m−1.K−1) |
| ρfg | Flue gas density (kg.m−3) |
| μfg | Flue gas dynamic viscosity (Pa.s) |
| η | Boiler efficiency (%) |
| δ | Iteration step of Runge-Kutta fourth order formula |
| Dimensionless numbers | |
| Re | Reynolds number |
| Pr | Prandtl number |
| Nu | Nusselt number |
References
- Demirbas, M.F.; Balat, M.; Balat, H. Potential contribution of biomass to the sustainable energy development. Energy Convers. Manag. 2009, 50, 1746–1760. [Google Scholar] [CrossRef]
- Sharma, A.; Pareek, V.; Zhang, D. Biomass pyrolysis—A review of modelling, process parameters and catalytic studies. Renew. Sustain. Energy Rev. 2015, 50, 1081–1096. [Google Scholar] [CrossRef]
- Sonnino, A. Agricultural biomass production is an energy option for the future. Renew. Energy 1994, 5, 857–865. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Soltani, R.; Dincer, I.; Rosen, M.A. Thermodynamic analysis of a novel multigeneration energy system based on heat recovery from a biomass CHP cycle. Appl. Therm. Eng. 2015, 89, 90–100. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Saidur, R.; Abdelaziz, E.; Demirbas, A.; Hossain, M.; Mekhilef, S. A review on biomass as a fuel for boilers. Renew. Sustain. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Strzalka, R.; Erhart, T.G.; Eicker, U. Analysis and optimization of a cogeneration system based on biomass combustion. Appl. Therm. Eng. 2013, 50, 1418–1426. [Google Scholar] [CrossRef]
- Li, C.; Gillum, C.; Toupin, K.; Donaldson, B. Biomass boiler energy conversion system analysis with the aid of exergy-based methods. Energy Convers. Manag. 2015, 103, 665–673. [Google Scholar] [CrossRef]
- Kang, S.B.; Kim, J.J.; Choi, K.S.; Sim, B.S.; Oh, H.Y. Development of a test facility to evaluate performance of a domestic wood pellet boiler. Renew. Energy 2013, 54, 2–7. [Google Scholar] [CrossRef]
- Gómez, M.A.; Porteiro, J.; de la Cuesta, D.; Patiño, D.; Míguez, J.L. Dynamic simulation of a biomass domestic boiler under thermally thick considerations. Energy Convers. Manag. 2017, 140, 260–272. [Google Scholar] [CrossRef]
- Zadravec, T.; Rajh, B.; Kokalj, F.; Samec, N. CFD modelling of air staged combustion in a wood pellet boiler using the coupled modelling approach. Therm. Sci. Eng. Prog. 2020, 20, 100715. [Google Scholar] [CrossRef]
- Karim, M.R.; Naser, J. CFD modelling of combustion and associated emission of wet woody biomass in a 4 MW moving grate boiler. Fuel 2018, 222, 656–674. [Google Scholar] [CrossRef]
- Tognoli, M.; Najafi, B. Dynamic modelling and optimal sizing of industrial fire-tube boilers for various demand profiles. Appl. Therm. Eng. 2018, 132, 341–351. [Google Scholar] [CrossRef]
- Bouvenot, J.-B.; Latour, B.; Siroux, M.; Flament, B.; Stabat, P.; Marchio, D. Dynamic model based on experimental investigations of a wood pellet. Appl. Therm. Eng. 2014, 73, 1039–1052. [Google Scholar] [CrossRef]
- Carlon, E.; Verma, V.K.; Schwarz, M.; Golicza, L.; Prada, A.; Baratieri, M.; Haslinger, W.; Schmidl, C. Experimental validation of a thermodynamic boiler model under steady state and dynamic conditions. Appl. Energy 2015, 138, 505–516. [Google Scholar] [CrossRef]
- Ziviani, D.; Beyene, A.; Venturini, M. Advances and challenges in ORC systems modeling for low grade thermal energy recovery. Appl. Energy 2014, 121, 79–95. [Google Scholar] [CrossRef]
- Féniès, G.; Formosa, F.; Ramousse, J.; Badel, A. Double acting Stirling engine: Modeling, experiments and optimization. Appl. Energy 2015, 159, 350–361. [Google Scholar] [CrossRef]
- Creyx, M.; Delacourt, E.; Morin, C.; Desmet, B. Dynamic modelling of the expansion cylinder of an open Joule cycle Ericsson engine: A bond graph approach. Energy 2016, 102, 31–43. [Google Scholar] [CrossRef]
- Lontsi, F.; Hamandjoda, O.; Fozao, K.; Stouffs, P.; Nganhou, J. Dynamic simulation of a small modified Joule cycle reciprocating Ericsson engine for micro-cogeneration systems. Energy 2013, 63, 309–316. [Google Scholar] [CrossRef]
- Gölles, M.; Reiter, S.; Brunner, T.; Dourdoumas, N.; Obernberger, I. Model based control of a small-scale biomass boiler. Control Eng. Pract. 2014, 22, 94–102. [Google Scholar] [CrossRef]
- Abdulmoneim, M.M.; Aboelela, M.A.; Dorrah, H.T. Hybrid modeling using power plant and controlling using fuzzy P+ID with application. Int. J. Adv. Eng. Technol. 2012, 4, 42–53. [Google Scholar]
- Paynter, H.M. Analysis and Design of Engineering Systems: Class Notes for M.I.T. Course; University of Michigan: Cambrige, MA, USA, 1961. [Google Scholar]
- Karnopp, D.; Rosenberg, R.C. System Dynamics: A Unified Approach; John Wiley & Sons Inc: Hoboken, NJ, USA, 1975. [Google Scholar]
- Merabtine, A.; Benelmir, R. Modeling of the RHC System with Bond Graphs Approach. Int. J. Therm. Environ. Eng. 2013, 5, 145–153. [Google Scholar]
- Nur Aziz, A.; Nazaruddin, Y.Y.; Siregar, P.; Bindar, Y. Structured Mathematical Modeling of Industrial Boiler. J. Eng. Technol. Sci. 2014, 46, 102–122. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; El-Bakkali, A.; Descombes, G.; Feidt, M.; Périlhon, C. Association of Finite-Time Thermodynamics and a Bond-Graph Approach for Modeling an Endoreversible Heat Engine. Entropy 2012, 14, 642–653. [Google Scholar] [CrossRef] [Green Version]
- Aridhi, E.; Abbes, M.; Mami, A. Pseudo bond graph model of a thermo-hydraulic system. In Proceedings of the International Conference on Modeling, Simulation and Applied Optimization, Hammamet, Tunisia, 28–30 April 2013; pp. 1–5. [Google Scholar]
- Couenne, F.; Jallut, C.; Maschke, B.; Breedveld, P.C. Bond graph for dynamic modelling in chemical engineering. Chem. Eng. Process. 2008, 47, 1994–2003. [Google Scholar] [CrossRef]
- Ould Bouamama, B.; el Harabi, R.; Abdelkrim, M.N.; Gayed, M.B. Bond Graphs for diagnosis of Chemical Processes. Comput. Chem. Eng. 2012, 36, 301–324. [Google Scholar] [CrossRef]
- Verma, V.K.; Bram, S.; Delattin, F.; de Ruyck, J. Real life performance of domestic pellet boiler technologies as a function of operational loads: A case study of Belgium. Appl. Energy 2013, 101, 357–362. [Google Scholar] [CrossRef]
- Åström, K.J.; Bell, R.D. Simple Drum-Boiler Models. IFAC Proc. Vol. 1988, 21, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Sandberg, J.; Fdhila, R.B.; Dahlquist, E.; Avelin, A. Dynamic simulation of fouling in a circulating fluidized biomass-fired boiler. Appl. Energy 2011, 88, 1813–1824. [Google Scholar] [CrossRef]
- Persson, T.; Fiedler, F.; Nordlander, S.; Bales, C.; Paavilainen, J. Validation of a dynamic model for wood pellet boilers and stoves. Appl. Energy 2009, 2009, 645–656. [Google Scholar] [CrossRef]
- Shannon, K.S.; Butler, B.W. A review of error associated with thermocouple temperature measurement in fire environments. In Proceedings of the 2nd International Wildland Fire Ecology and Fire Management Congress and the 5th Symposium on Fire and Forest Meteorology, Orlando, FL, USA, 16–20 November 2003. [Google Scholar]
- Hindasageri, V.; Vedula, R.P.; Prabhu, V. Thermocouple error correction for measuring the flame temperature with determination of emissivity and heat transfer coefficient. Rev. Sci. Instrum. 2013, 84, 024902-1–024902-11. [Google Scholar] [CrossRef] [PubMed]
- Winterton, R.H. Where did the Dittus and Boelter equation come from? Int. J. Heat Mass Transf. 1998, 41, 809–810. [Google Scholar] [CrossRef]
- Gnielinski, V. Heat Transfer Coefficients for Turbulent Flow in Concentric Annular Ducts. Heat Transf. Eng. 2009, 30, 431–436. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. The Properties of Gases and Liquids, 5th ed.; The McGraw-Hill Companies: Henrico, VA, USA, 2001. [Google Scholar]
- Krieager, F.J. Calculation of the viscosity gas mixtures. RAND Corp. 1951, RM-649, 1–11. [Google Scholar]
- Stull, D.R.; Prophet, H. JANAF Thermochemical Tables; Defense Technical Information Center: Washington, DC, USA, 1971; pp. 1856–1985.
- Rajaraman, V. Computer Oriented Numerical Methods, 3rd ed.; Prentice-Hall of India: New Delhi, India, 2006. [Google Scholar]
- Najafi-Yazdi, A.; Mongeau, L. A low-dispersion and low-dissipation implicit Runge–Kutta scheme. J. Comput. Phys. 2013, 233, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Akanbi, M.A.; Okunuga, S.A.; Okunuga, A.B. Runge-Kutta Schemes for Solving Electrical Network Problems. J. Sci. Res. Dev. 2001, 6, 31–44. [Google Scholar]
- Kim, D.; Stanescu, D. Low-storage Runge–Kutta methods for stochastic differential equations. Appl. Numer. Math. 2008, 58, 1479–1502. [Google Scholar] [CrossRef]









| Reference | Device | Study | Power | Main Objective |
|---|---|---|---|---|
| Strzalka et al. [8] | Biomass grate furnace | Mathematical modeling | 6 kW | Model-based optimization of control strategies of grate furnaces. |
| Li et al. [9] | Biomass boiler | Thermodynamic modeling | Conventional exergy analysis and advanced exergy analysis of a real biomass boiler. | |
| Kang et al. [10] | Biomass boiler | Experimental investigation | 24 kW | Evaluation of the performances of a domestic wood pellet boiler. |
| Gómez et al. [11] | Biomass domestic boiler | CFD modeling | 27 kW | Simulation of the boiler operation under transient conditions. The effect of the parameters influencing the combustion process has been studied. |
| Ziviani et al. [17] | ORC system | Dynamic modeling (AMESim) | Progress and challenges related to the operation of ORC (Organic Rankine Cycle) systems. | |
| Féniès et al. [18] | Stirling engine | Theoretical modeling and experimental study | 18 W | Establishment of two models, thermal and electrical, and study of the influence of dead volume, the natural frequency of mechanical oscillations and thermal conduction between the hot and cold sides for engine optimization. |
| Abdulmoneim et al. [22] | Thermal power generation station | Dynamic modeling (Bond Graph) | Modeling of hybrid power plant: pump, boiler, economizer, evaporator, super heater, drum and pipe. | |
| Creyx et al. [19] | Ericsson engine | Dynamic modeling (Bond Graph) | Dynamic model of the expansion cylinder of an open Joule cycle Ericsson engine. | |
| Ould-Bouamama et al. [30] | Chemical reactor | Dynamic modeling (Bond Graph) | Modeling of a chemical reactor for monitoring. | |
| Sandberg et al. [33] | Biomass boiler | Dynamic modeling | 157 MW | Biomass boiler dynamic model. |
| Persson et al. [34] | Biomass boiler and stove | Dynamic modeling (TRNSYS) | 10 kW | Development and validation of a dynamic boiler/pellet stove model based on experimental measurements. |
| Location | Flow Configuration | Correlations | Valid Range |
|---|---|---|---|
| Combustion chamber and flue gas tubes [37] | Inside a cylinder | 0.7 ≤ Pr ≤ 120 ≤ 1.2 105 ≤ 20 | |
| Passage between the combustion chamber and inner wall of the heat exchanger [38] | Inside an annular duct–fixed walls | 0.7 < Pr < 100 > 2000 |
| Flue Gas Thermodynamic Properties | Correlations | Units | Temperature Range (K) | Min–Max |
|---|---|---|---|---|
| Density | kg.m−3 | 298–1500 | 0.23–1.22 | |
| Thermal conductivity [39] | W.m−1.K−1 | 298–1500 | 2.32 10−2–8.65 10−2 | |
| Dynamic viscosity [40] | Pa.s−1 | 298–1500 | 1.711 10−5–5.42 10−5 | |
| Specific heat [41] | J.kg−1.K−1 | 298–1500 | 1090–1374 |
| Location | ϕrad/ϕtot (%) |
|---|---|
| Inside the combustion chamber | 97.6 |
| Outside the combustion chamber (annular passage) | 96.8 |
| Inside the heat exchanger (flue gas side) | 96.1 |
| Inside the flue gas pipes | 95.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mameri, F.; Delacourt, E.; Morin, C.; Schiffler, J. 0D Dynamic Modeling and Experimental Characterization of a Biomass Boiler with Mass and Energy Balance. Entropy 2022, 24, 202. https://doi.org/10.3390/e24020202
Mameri F, Delacourt E, Morin C, Schiffler J. 0D Dynamic Modeling and Experimental Characterization of a Biomass Boiler with Mass and Energy Balance. Entropy. 2022; 24(2):202. https://doi.org/10.3390/e24020202
Chicago/Turabian StyleMameri, Fateh, Eric Delacourt, Céline Morin, and Jesse Schiffler. 2022. "0D Dynamic Modeling and Experimental Characterization of a Biomass Boiler with Mass and Energy Balance" Entropy 24, no. 2: 202. https://doi.org/10.3390/e24020202





