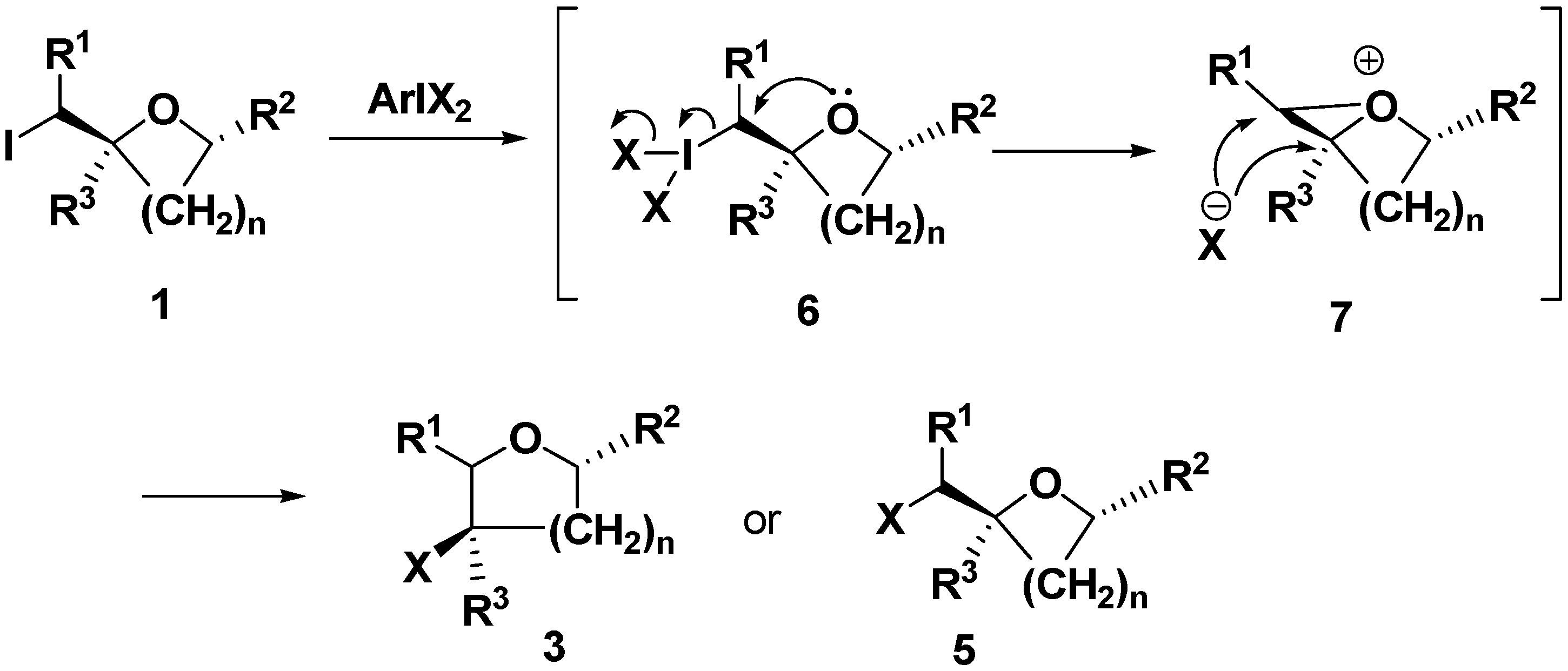
General
1H-NMR (400MHz) and
13C-NMR (100MHz) spectra were recorded in CDCl
3 on a JEOL JNM-A400II FT NMR and the chemical shift, δ, is referred to TMS. The EI-low and high-resolution mass spectra were measured on a JEOL JMS-700TZ, JMS-FABmate or JMS-HX110. DIT was prepared from iodotoluene according to the literature [
14]. BTI was obtained from Sigma-Aldrich Co. and used without further purification.
(2R*, 3S*)-3-Acetoxy-2-octyl-3-methyltetrahydropyran (3a). To DIT (370 mg, 1.1 mmol) in a mixture of HFIP (1 mL) and CH2Cl2 (1 mL), was added a CH2Cl2 solution (1 mL) of 1a (324 mg, 1 mmol) at room temperature and the mixture was stirred at the temperature for 1 h. Water (5 mL) and ether (5 mL) were added to the reaction mixture and the separated aqueous layer was extracted with ether (3 x 5 mL). The combined organic layer was washed with aqueous Na2S2O3, aqueous NaHCO3, and brine, successively. Then, the organic layer was dried over MgSO4, and concentrated under reduced pressure. Purification by column chromatography (silica gel / hexane-ether) gave 3a (217 mg, 80 % ). 1H-NMR δ: 3.94 – 3.90 (1H, m), 3.43 – 3.37 (1H, m), 3.29 (1H, d, J = 8.1 Hz), 2.65 – 2.62 (1H, m), 1.98 (3H, s), 1.77 – 1.52 (5H, m), 1.48 (3H, s), 1.28 (12H, brs), 0.88 (3H, t, J = 7.1 Hz); 13C=NMR δ: 14.1, 17.3, 22.4, 22.7, 24.3, 26.5, 28.8, 29.3, 29.6, 29.7, 31.9, 35.0, 63.8, 80.8, 82.1, 170.1; HRMS (EI) Calc. for C16H31O3 (M++H) 271.2273. Found: 271.2281.
The formation of ca. 2% of 2-(2-acetoxynonyl)-2-methyltetrahydrofuran (5a) was confirmed by GC. 1H-NMR δ: 4.91 (1H, dd, J = 10.5, 2.0 Hz), 3.89 – 3.84 (1H, m), 3.81 - 3.75 (1H, m), 2.08 (3H, s), 1.93 – 1.83 (3H, m), 1.64 – 1.41 (3H, m), 1.25 (12H, brs), 1.16 (3H, s), 0.88 (3H, t, J = 6.6 Hz); 13C-NMR δ: 14.1, 21.1, 22.5, 22.6, 26.0, 26.1, 29.2, 29.5, 29.6, 29.7, 31.8, 34.5, 68.3, 76.7, 83.7, 170.9; HRMS (EI) Calc. for C16H31O3 (M++H) 271.2273. Found: 271.2258.
(2R*, 5R*)-5-Acetoxy-2-hexyl-5-methyltetrahydropyran (
3b).
1H-NMR δ: 3.91 (1H, dd, J = 11.0, 2.4 Hz), 3.38 (1H, d, J = 11.0 Hz), 3.27 (1H, m), 2.38 – 2.32 (1H, m), 1.98 (3H, s), 1.59 (3H, s), 1.77 – 1.27 (13H, m), 0.88 (3H, t, J = 7.1 Hz);
13C-NMR δ: 14.1, 20.8, 22.2, 22.6, 25.6, 29.2, 29.3, 31.8, 34.4, 35.5, 73.9, 78.0, 78.3, 170.1; HRMS (EI) Calc. for C
14H
26O
3 (M
+) 242.1882. Found: 242.1878. The stereochemistry of
3b was determined by comparison of chemical shifts in
1H-NMR with reported data [
15].
(2R*, 5R*)-5-Acetoxy-2-hexyltetrahydropyran (
3c).
1H-NMR δ: 4.75 (1H, m), 4.00 (1H, ddd, J = 10.5, 4.9, 2.2 Hz), 3.25 – 3.12 (2H, m), 2.16 – 2.12 (1H, m), 2.03 (3H, s), 1.76 – 1.27 (13H, m), 0.88 (3H, t, J = 7.1 Hz);
13C-NMR δ: 14.1, 21.1, 22.6, 25.6, 29.2, 29.3, 30.2, 31.8, 35.6, 68.5, 69.2, 77.5, 170.3; HRMS (EI) Calc. for C
13H
24O
3 (M
+) 228.1725. Found: 228.1709. The stereochemistry of
3c was determined by comparison of chemical shifts in
1H-NMR with reported data [
16].
(2R*, 5S*)-5-Acetoxymethyl-2-hexyltetrahydrofuran (5c). 1H-NMR δ: 4.26 – 3.89 (2H, m), 2.10 (3H, s), 2.09 – 2.00 (2H, m), 1.65 -1.37 (14H, m), 0.88 (3H, t, J = 6.8 Hz).
(2R*, 5S*)-5-Acetoxy-2-hexyltetrahydropyran (
3d).
1H-NMR δ: 4.80 (1H, brs), 4.01 (1H, d, J = 12.9 Hz), 3.58 (1H, dd, J = 12.9, 1.7 Hz), 3.31– 3.26 (1H, m), 2.11 (3H, s), 2.09 – 1.94 (1H, m), 1.78 – 1.28 (13H, m), 0.88 (3H, t, J = 7.1 Hz);
13C-NMR δ: 14.1, 21.4, 22.6, 25.5, 26.7, 27.4, 29.3, 31.8, 36.2, 67.5, 69.7, 77.7, 170.9; HRMS (EI) Calc. for C
13H
24O
3 (M
+) 228.1725. Found: 228.1723. The stereo- chemistry of
3d was determined by comparison of its
1H-NMR chemical shifts with reported data [
16].
(2R*, 5R*)-5-Acetoxymethyl-2-hexyltetrahydrofuran (5d). 1H-NMR δ: 4.19 – 3.85 (2H, m), 2.09 (3H, s), 1.93 – 1.88 (2H, m), 1.68 – 1.37 (14H, m), 0.88 (3H, t, J = 6.8 Hz).
(2R*, 4R*)-4-Trifluoroacetoxy-2-heptyl-4-methyltetrahydrofuran (4e). 1H-NMR δ: 3.94 (1H, d, J = 7.1 Hz), 3.56 (1H, dd, J = 7.3, 1.5 Hz), 2.26 (1H, ddd, J = 13.7, 6.6, 1.5 Hz), 1.79 (2H, dd, J = 13.9, 7.1 Hz), 1.53 (3H, s), 1.45 – 1.43 (2H, m), 1.28 (11H, brs), 0.88 (3H, t, J = 7.1 Hz); 13C-NMR δ: 14.0, 21.8, 22.6, 25.5, 29.1, 29.3, 31.7, 35.6, 41.2, 69.5, 75.1, 79.6, 117.5, 120.4; HRMS (EI) Calc. for C14H24O3 F3 (M+) 296.1599. Found: 296.1603. The stereochemistry of 4e was determined from a NOESY experiment.
(2R*, 4S*)-4-Trifluoroacetoxy-2-heptyl-4-methyltetrahydrofuran (4f). 1H-NMR δ: 4.19 – 4.13 (1H, m), 4.10 (1H, d, J = 7.1 Hz), 3.66 (1H, d, J = 7.1 Hz), 1.46 (3H, s), 1.78 – 1.27 (14H, m), 0.88 (3H, t, J = 7.1 Hz); 13C-NMR δ: 14.1, 21.3, 22.6, 24.7, 29.1, 29.3, 31.7, 35.1, 39.9, 70.4, 74.2, 80.7, 117.3, 120.1; HRMS (EI) Calc. for C14H24O3F3 (M+) 296.1599. Found: 296.1603. The stereochemistry of 4f was determined from a NOESY experiment.
6-Acetoxy-2-hexyl-6-methyloxepane (3g). 1H-NMR δ: 4.24 (1H, d, J = 13.7 Hz), 3.36– 3.30 (1H, m), 3.25 (1H, d, J = 13.7 Hz), 2.13 – 2.03 (2H, m), 2.01 (3H, s), 1.85 – 1.70 (2H, m), 1.40 (3H, s), 1.58 – 1.26 (12H, m), 0.88 (3H, t, J = 7.1 Hz); 13C-NMR δ: 14.1, 20.5, 21.5, 22.5, 22.6, 26.1, 29.3, 31.8, 36.6, 36.7, 38.2, 77.2, 83.6, 85.7, 170.7; HRMS (EI) Calc. for C15H28O3 (M+) 256.2038. Found: 256.2038. Only a single stereoisomer was contained in 3g, however the identification of its stereochemistry failed.