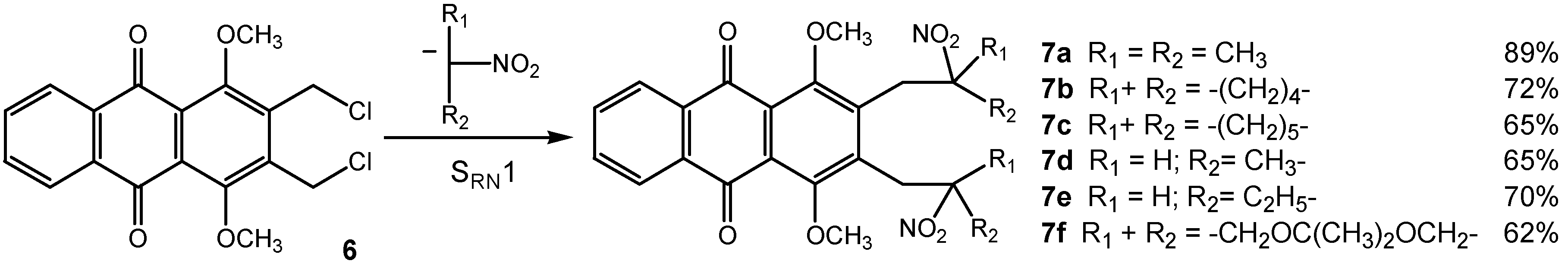
General procedure for bis-SRN1 reaction with aliphatic and cyclic nitronate anions.
Under a nitrogen atmosphere, a solution of tetrabutylammonium hydroxide (40% in water, 2.1 mL, 2.75 mmol) was treated with nitroalkane (2.75 mmol) for 1 h. A solution of 2,3-bis(chloromethyl)-1,4-dimethoxyanthraquinone (6, 0.20 g, 0.55 mmol) in dichloromethane (20 mL) was then added and the mixture was irradiated with a 300W sun lamp for 20 min at room temperature under an inert atmosphere. The organic layer was separated and the aqueous layer was extracted with dichloro-methane (3 x 10 mL). The combined organic layers were washed twice with water (30 mL), dried over MgSO4 and removed under reduced pressure. Purification by chromatography on silica gel eluting with chloroform and recrystallization from ethanol led to the corresponding products 7a-f.
1,4-Dimethoxy-2,3-bis(2-methyl-2-nitropropyl)anthraquinone (7a): Orange solid, mp 168.4°C (ethanol); 1H-NMR δ: 1.55 (s, 12H, 4xCH3), 3.37 (s, 4H, CH2), 3.83 (s, 6H, 2xOCH3), 7.74-7.77 (m, 2H, 2xAr-H), 8.17-8.20 (m, 2H, 2xAr-H); 13C-NMR δ: 26.1 (4xCH3), 37.0 (2xCH2), 62.0 (2xOCH3), 88.6 (2xCNO2), 125.7 (2xC), 126.7 (2xCH), 133.8 (2xCH), 133.9 (2xC), 139.4 (2xC), 156.2 (2xC), 182.4 (2xC=O); Anal. Calcd for C24H26N2O8: C, 61.27; H, 5.57; N, 5.95. Found: C, 61.42; H, 5.60; N, 5.78.
1,4-Dimethoxy-2,3-bis(1-nitrocyclopentylmethyl)anthraquinone (7b): Orange solid, mp 98°C (ethanol); 1H-NMR δ: 1.58-1.69 (m, 8H, 4xCH2), 1.79-1.90 (m, 4H, 2xCH2), 2.49-2.60 (m, 4H, 2xCH2), 3.47 (s, 4H, 2xCH2), 3.85 (s, 6H, 2xOCH3), 7.74-7.78 (m, 2H, 2xAr-H), 8.18-8.22 (m, 2H, 2xAr-H); 13C-NMR δ: 22.7 (4xCH2), 34.7 (2xCH2), 36.3 (4xCH2), 62.1 (2xOCH3), 101.1 (2xCNO2), 125.9 (2xC), 126.7 (2xCH), 133.8 (2xCH), 134.0 (2xC), 139.9 (2xC), 156.1 (2xC), 182.5 (2xC=O); Anal. Calcd for C28H30N2O8: C, 64.36; H, 5.79; N, 5.36. Found: C, 64.27; H, 5.77; N, 5.17.
1,4-Dimethoxy-2,3-bis(1-nitrocyclohexylmethyl)anthraquinone (7c): Yellow solid, mp 191°C (ethanol); 1H-NMR δ: 1.12-1.27 (m, 4H, 2xCH2), 1.59-1.67 (m, 12H, 6xCH2), 2.31-2.45 (m, 4H, 2xCH2), 3.18 (s, 4H, 2xCH2), 3.84 (s, 6H, 2xOCH3), 7.73-7.77 (m, 2H, 2xAr-H), 8.17-8.21 (m, 2H, 2xAr-H); 13C-NMR δ: 22.2 (6xCH2), 24.3 (2xCH2), 34.2 (2xCH2), 37.5 (2xCH2), 61.9 (2xOCH3), 92.5 (2xCNO2), 125.4 (2xC), 126.6 (2xCH), 133.7 (2xCH), 134.0 (2xC), 139.2 (2xC), 156.0 (2xC), 182.5 (2xC=O); Anal. Calcd for C30H34N2O8: C, 65.44; H, 6.22; N, 5.09. Found: C, 65.48; H, 6.22; N, 4.85.
1,4-Dimethoxy-2,3-bis(2-nitropropyl)anthraquinone (7d): Orange solid, mp 185.9°C (ethanol); 1H‑NMR δ: 1.58 (d, 3H, J = 6.7 Hz), 1.68 (d, 3H, J = 6.7 Hz), 3.19-3.54 (m, 4H, 2xCH2), 3.90 (s, 6H, 2xOCH3), 4.91-5.02 (m, 2H, 2xCHNO2), 7.75-7.79 (m, 2H, 2xAr-H), 8.18-8.22 (m, 2H, 2xAr-H); 13C‑NMR δ: 19.5 (2xCH3), 33.0 (2xCH2), 62.4 (2xOCH3), 82.9 (2xCHNO2), 125.9 (2xC), 126.7 (2xCH), 133.9 (2xCH), 138.8 (2xC), 139.4 (2xC), 156.0 (2xC), 182.3 (2xC=O); Anal. Calcd for C22H22N2O8: C, 59.73; H, 5.01; N, 6.33. Found: C, 59.74; H, 4.84; N, 6.39.
1,4-Dimethoxy-2,3-bis(2-nitrobutyl)anthraquinone (7e): Orange solid, mp 145.8 °C (ethanol); 1H NMR δ: 1.00 (t, 3H, J = 7.4 Hz), 1.05 (t, 3H, J = 7.4 Hz), 1.81-2.18 (m, 4H, 2xCH2), 3.22-3.45 (m, 4H, 2xCH2), 3.90 (s, 6H, 2xOCH3), 4.74-4.83 (m, 2H, 2xCHNO2), 7.75-7.78 (m, 2H, 2xAr-H), 8.18-8.21 (m, 2H, 2xAr-H); 13C NMR δ: 10.2 (2xCH3), 27.9 (2xCH2), 31.4 (2xCH2), 62.3 (2xOCH3), 89.3 (2xCHNO2), 125.7 (2xC), 126.7 (2xCH), 133.8 (2xC), 133.9 (2xCH), 139.5 (2xC), 155.9 (2xC), 182.4 (2xC=O); Anal. Calcd for C24H26N2O8: C, 61.27; H, 5.57; N, 5.95. Found: C, 61.15; H, 5.74; N, 5.95.
2,3-Bis(2,2-dimethyl-5-nitro[1,3]dioxan-5-ylmethyl)-1,4-dimethoxyanthraquinone (7f): Yellow solid, mp 215 °C (ethanol); 1H-NMR δ: 1.35 (s, 6H, 2xCH3), 1.50 (s, 6H, 2xCH3), 3.39 (s, 4H, 2xCH2), 3.85 (s, 6H, 2xOCH3), 4.04 (d, 4H, JAB = 12.8 Hz, 2xCH2O), 4.31 (d, 4H, JAB = 12.8 Hz, 2xCH2O), 7.75-7.80 (m, 2H, 2xAr-H), 8.17-8.22 (m, 2H, 2xAr-H); 13C-NMR δ: 21.9 (2xCH3), 24.7 (2xCH3), 30.5 (2xCH2), 61.4 (2xCH2O), 62.3 (2xOCH3), 63.9 (2xCH2O), 85.2 (2xCNO2), 99.0 (2xC), 126.0 (2xC), 126.7 (2xCH), 133.9 (2xCH), 134.0 (2xC), 137.4 (2xC), 156.1 (2xC), 182.2 (2xC=O); Anal. Calcd for C30H34N2O12: C, 58.63; H, 5.58; N, 4.56. Found: C, 58.76; H, 5.69; N, 4.38.
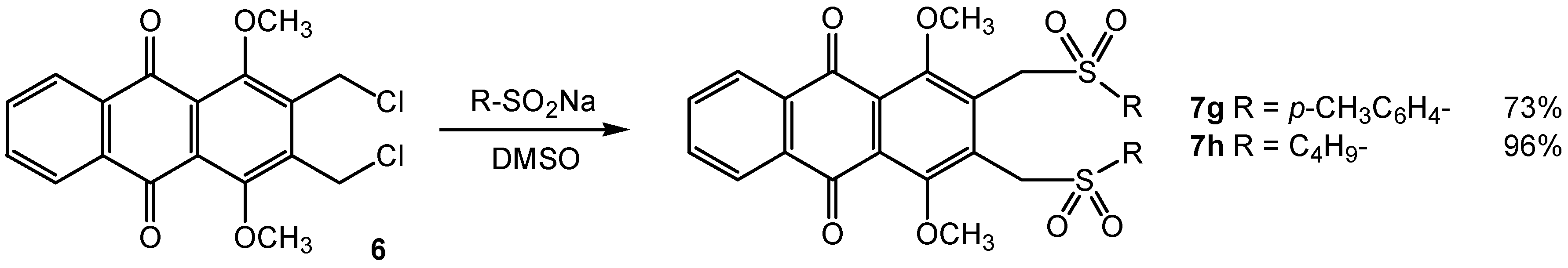
General procedure for reactions with substituted sulfinate sodium salt
A solution of substituted sulfinate sodium salt (3.3 mmol) in dimethylsulfoxide (10 mL) was added dropwise to a solution of dichloride 6 (0.20 g, 0.55 mmol) in dimethylsulfoxide (6 mL) and stirred under inert atmosphere for 10 min. The reaction mixture was poured into cold water and a precipitate was formed. After filtration, the crude product was recrystallized from the corresponding solvent gave the corresponding bis-S-alkylated product.
1,4-Dimethoxy-2,3-bis-(toluene-4-sulfonylmethyl)anthraquinone (7g): Yellow solid, mp 281°C (ethyl acetate); 1H-NMR δ: 2.42 (s, 6H, 2xCH3), 3.90 (s, 6H, 2xOCH3), 5.07 (s, 4H, 2xCH2SO2), 7.34 (d, 4H, J = 8.2 Hz, 4xAr-H), 7.74 (d, 4H, J = 8.2 Hz, 4xAr-H), 7.76-7.80 (m, 2H, 2xAr-H), 8.17-8.22 (m, 2H, 2xAr-H); 13C-NMR δ: 21.6 (2xCH3), 54.3 (2xCH2SO2), 63.6 (2xOCH3), 126.7 (2xCH), 128.1 (4xCH), 130.0 (4xCH), 132.4 (2xCH), 133.8 (2xC), 134.0 (2xC), 145.3 (2xC), 156.7 (2xC), 182.0 (2xC=O); Anal. Calcd for C32H28O8S2: C, 63.56; H, 4.67. Found: C, 63.65; H, 4.67.
2,3-Bis(butane-1-sulfonylmethyl)-1,4-dimethoxyanthraquinone (7h): Yellow solid, mp 184°C (ethanol); 1H-NMR δ: 0.96 (t, 6H, J = 6.6 Hz, 2xCH3), 1.43-1.55 (m, 4H, 2xCH2), 1.82-1.94 (m, 4H, 2xCH2), 3.04-3.12 (m, 4H, 2xCH2), 3.99 (s, 6H, 2xOCH3), 4.98 (s, 4H, 2xCH2SO2), 7.77-7.81 (m, 2H, 2xAr-H), 8.19-8.23 (m, 2H, 2xAr-H); 13C-NMR δ: 13.5 (2xCH3), 21.7 (2xCH2), 24.0 (2xCH2), 50.6 (2xCH2SO2), 53.6 (2xCH2SO2), 63.6 (2xOCH3), 126.8 (2xCH), 127.3 (2xC), 132.7 (2xC), 133.5 (2xC), 134.1 (2xCH), 156.3 (2xC), 182.1 (2xC=O); Anal. Calcd for C26H32O8S2: C, 58.19; H, 6.01. Found: C, 58.23; H, 5.84.