Diastereoselective Synthesis of 2-Phenylselenenyl-1,3-anti-Diols and 2-Phenylselenenyl-1,3-anti-Azido-Alcohols via Hydroxyand Azido-Selenenylation Reactions
Abstract
:Introduction
Results and Discussion

| 1 | R1 | R2 | 1 | R1 | R2 |
|---|---|---|---|---|---|
| a | Me | Ph | e | Ph | Ph |
| b | i-Pr | Ph | f | EtOCOCH2 | Ph |
| c | Me | p-Cl-Ph | g | Me | CH2Ph |
| d | Me | Me |

| Entry | Compd. | 2+3 (%) | 1 (%) | 2:3 |
|---|---|---|---|---|
| 1 | 1a | 88 | 11 | 90 :10 |
| 2 | 1b | 69 | 32 | 95 :5 |
| 3 | 1c | 63 | 24 | 92 :8 |
| 4 | 1d | 55 | 40 | 91 :9 |
| 5 | 1e | 77 | 11 | 94:6 |
| 6[4a] | 1f | 80 | <5% | 95:5 |
| Entry | PhSeX | Solvent | 4+5 (%) | 1 (%) | 4/5 |
|---|---|---|---|---|---|
| 1a | PhSeCl | DMSO | 50 | 8 | 86/14 |
| 2 b | PhSeCl | DMSO | 66 | 7 | 84/16 |
| 3 c | PhSeCl | DMSO | 46 | 18 | 85/15 |
| 4 b | PhSeCl | DMF | 20 | 45 | 81/19 |
| 5 b | PhSeCl | MeCN | 32 | 40 | 88/12 |
| 6 b | PhSeCl | DME | 1 | 99 | - |
| 7 b | PhSeOTf | MeCN | 43 | 29 | 87/13 |
| 8 b | PhSeOTf | DMSO | 73 | 10 | 87/13 |
| 9b | PhSeCl | bmimBF4 | 24 | 20 | 81/19 |
| Entry | Compd. | 4+5 | 1 | 4/5 |
|---|---|---|---|---|
| 1 | 1a | 73 | 10 | 87:13 |
| 2 | 1b | 51 | 45 | 84:16 |
| 3 | 1c | 64 | 30 | 83:17 |
| 4 | 1d | 58 | 40 | 92:8 |
| 5 | 1e | 33 | 33 | 87:13 |
| 6 | 1f | 48 | 43 | 82:18 |
| 7 | 1g | 57 | 35 | 93:7 |
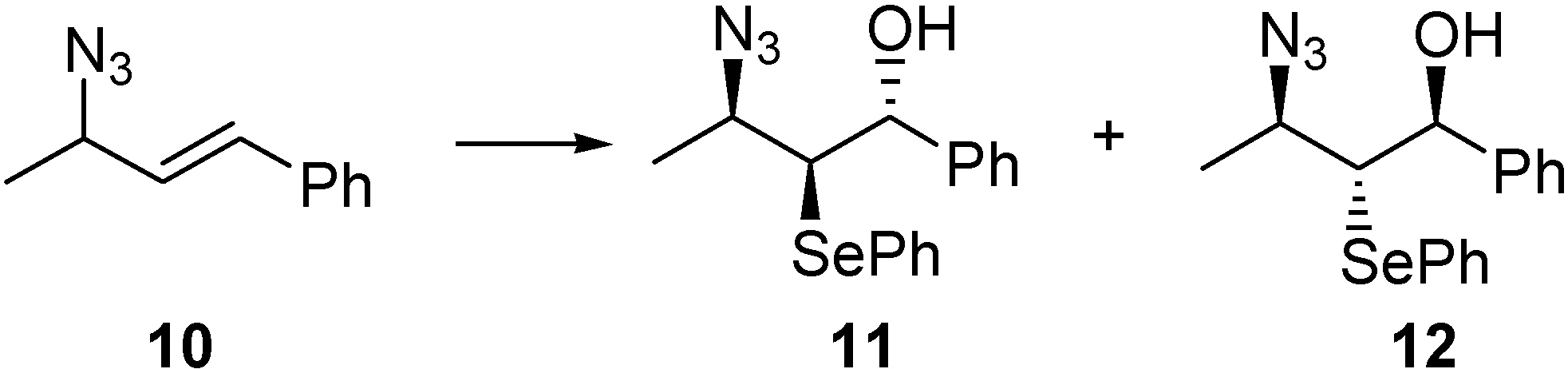
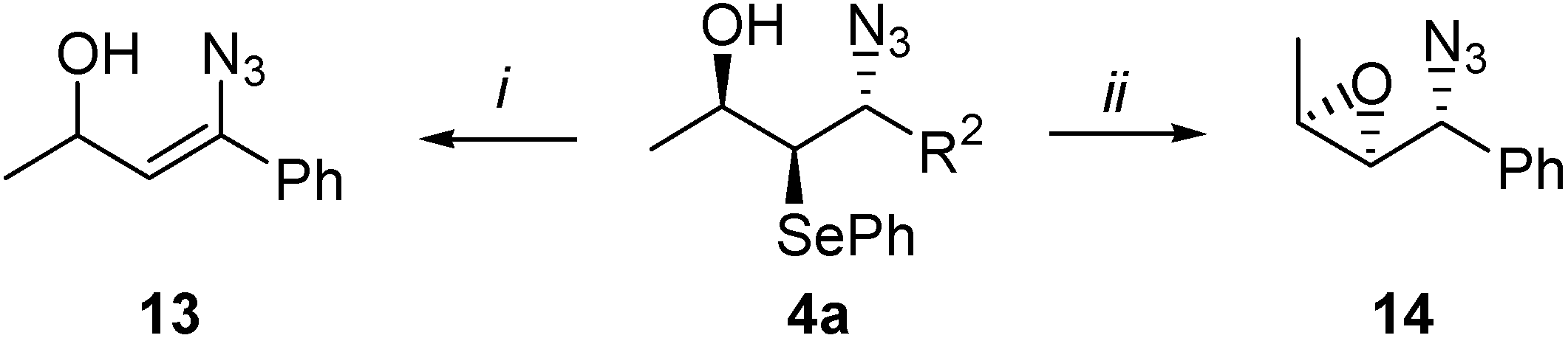
Conclusions
Acknowledgments
Experimental
General
General procedure for hydroxy-selenenylation reactions:
General procedure for azido-selenenylation reactions:
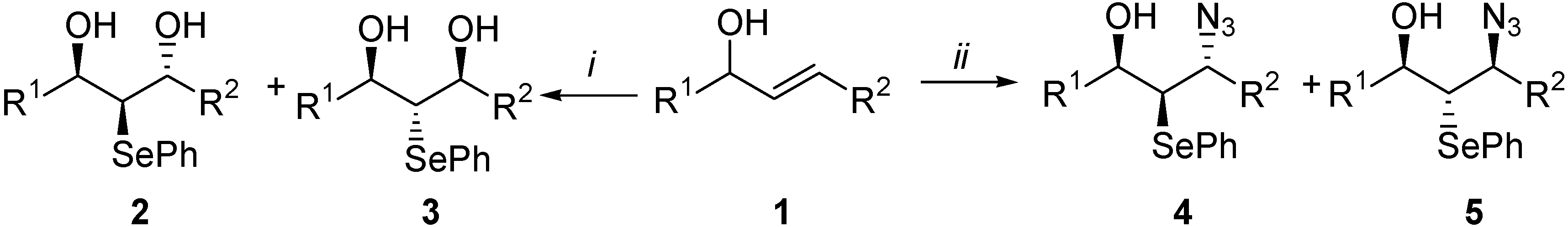
(±)-(4RS, 5RS, 6RS)-6-methyl-4-phenyl-5-phenylselenenyl-1,3-oxazolidin-2-one (9)
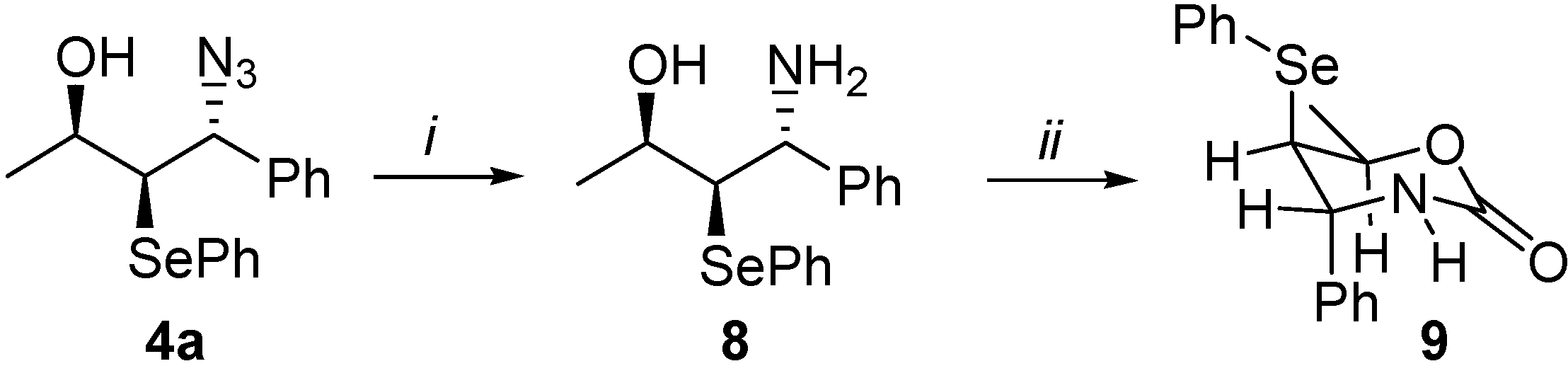
Hydroxy-selenenylation reaction of compound 10
Oxidation of compound 4a
References and Notes
- Rychnovsky, S.D. Oxo polyene macrolide antibiotics. Chem. Rev. 1995, 95, 2021–2040. [Google Scholar] [CrossRef]
- See for example: Oishi, T.; Nakata, T. New aspects of stereoselective synthesis of 1,3-polyols. Synthesis 1990, 635–645. [Google Scholar] Enders, D.; Hundertmark, T.; Lampe, C.; Jegelka, U.; Scharfbillig, I. Highly diastereo- and enantioselective synthesis of protected anti-1,3-diols. Eur. J. Org. Chem. 1998, 2839–2849, and references cited therein. [Google Scholar]
- Toujas, J.-L.; Toupet, L.; Vaultier, M. Organometallic additions to β-substituted N-Boc-β-aminoaldehydes: a new synthesis of enantiomerically pure 1,3-disubstituted N-Boc-1,3-aminoalcohols. Tetrahedron 2000, 56, 2665–2672, and references cited therein. [Google Scholar]
- Aprile, C.; Gruttadauria, M.; Amato, M.E.; D’Anna, F.; Lo Meo, P.; Riela, S.; Noto, R. Studies on the stereoselective selenolactonization, hydroxy and methoxy selenenylation of α- and β-hydroxy acids and esters. Synthesis of δ- and γ-lactones. Tetrahedron 2003, 59, 2241–2251. [Google Scholar] Gruttadauria, M.; Aprile, C.; Lo Meo, P.; Riela, S.; Noto, R. Diastereoselective synthesis of substituted 2-phenyltetrahydropyrans as useful precursors of aryl C-glycosides via selenoetherification. Heterocycles 2003, 63, 681–690, and references cited therein. [Google Scholar]
- Wirth, T. (Ed.) “Organoselenium Chemistry, Modern Developments in Organic Synthesis”. Top. Curr. Chem. 2004, 208.
- Only few examples are reported: Cooper, M.A.; Ward, A.D. Hydroxyselenation of allylic alcohols. Tetrahedron Lett. 1995, 36, 2327–2330. [Google Scholar] Haughan, A.F.; Knight, J.R.; Sweeney, J.B. Hydroxyselenation of acetoxycyclohex-2-ene. Tetrahedron Lett. 1994, 35, 1781–1784. [Google Scholar]
- Tiecco, M.; Testaferri, L.; Temperini, A.; Bagnoli, L.; Marini, F.; Santi, C. Electrophilic azido selenenylation of alkenes. A simple synthetic route to racemic taxol side chain. Synthetic Commun. 1998, 28, 2167–2179. [Google Scholar] Tiecco, M.; Testaferri, L.; Santi, C.; Tomassini, C.; Marini, F.; Bagnoli, L.; Temperini, A. Asymmetric azidoselenenylation of alkenes: a key step for the synthesis of enantiomerically enriched nitrogen-containing compounds. Angew. Chem. Int. Ed. 2003, 42, 3131–3133. [Google Scholar]
- Hassner, A.; Amarasekara, A.S. Phenylselenium azide addition to alkenes. A new and stereospecific introduction of Se and N into organic molecules. Tetrahedron Lett. 1987, 28, 5185–5188. [Google Scholar]
- Tingoli, M.; Tiecco, M.; Chianelli, D.; Balducci, R.; Temperini, A. Novel azido-phenylselenenylation of double bonds. Evidences for a free radical process. J. Org. Chem. 1991, 56, 6809–6813. [Google Scholar]
- Ponthieux, S.; Outurquin, F.; Paulmier, C. Cross-aldol reaction between benzaldehyde and β-phenylselanyl enoxysilanes derived from phenylselanylmethylketones. Tetrahedron Lett. 1998, 39, 4017–4020. [Google Scholar] [CrossRef]
- Hori, T.; Sharpless, K.B. Synthetic applications of arylselenenic and arylseleninic acids. Conversion of olefins to allylic alcohols and epoxides. J. Org. Chem. 1978, 43, 1689–1696. [Google Scholar]
- This reaction takes place because of the small amount of water contained in the bmimBF4 ionic liquid.
- It is interesting to note that the diastereomeric ratio observed with the TBDMS derivative of compound 1a was still high (91:9).
- Padwa, A.; Sá, M.M. Intramolecular O-H insertion reaction of azido substituted diazoesters and its relevance to the mechanism of the allylic azide rearrangement. Tetrahedron Lett. 1997, 38, 5087–5090. [Google Scholar] Arimoto, M.; Yamaguchi, H.; Fujita, E. Iodosylbenzene-trimethylsilyl azide-boron trifluoride etherate: a highly efficient system for direct synthesis of allyl azides from allylsilanes. Tetrahedron Lett. 1987, 28, 6289–6292. [Google Scholar]
- Toshimitsu, A.; Uemura, S. Organoselenium Chemistry; Back, T.G., Ed.; Oxford University Press: Oxford, 1999; Chapter 13; p. 244. [Google Scholar] Uemura, S.; Ohe, K.; Sugita, N. Oxidative conversion of β-hydroxyselenides to epoxides and ketones with meta-chloroperbenzoic acid. J. Chem. Soc., Chem. Commun. 1988, 111–112. [Google Scholar] Krief, A.; Dumont, W.; Laboureur, J.L. Exploratory study on α-metallo selenones. Original syntheses of oxaspiropentanones. Tetrahedron Lett. 1988, 29, 3265–3268. [Google Scholar]
- Napolitano, E.; Fiaschi, R. Addition of iodine azide to allylic alcohols. Stereospecific synthesis of 3-azido-1,2-epoxides. Gazz. Chim. Ital. 1992, 122, 233–235. [Google Scholar]
- Murahashi, S.-I.; Taniguchi, Y.; Imada, Y.; Tanigawa, Y. Palladium(0)-catalyzed azidation of allyl esters. Selective synthesis of allyl azides, primary allylamines, and related compounds. J. Org. Chem. 1989, 54, 3292–3303. [Google Scholar]
- Sample availability: Available from the authors.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Riela, S.; Aprile, C.; Gruttadauria, M.; Meo, P.L.; Noto, R. Diastereoselective Synthesis of 2-Phenylselenenyl-1,3-anti-Diols and 2-Phenylselenenyl-1,3-anti-Azido-Alcohols via Hydroxyand Azido-Selenenylation Reactions. Molecules 2005, 10, 383-393. https://doi.org/10.3390/10020383
Riela S, Aprile C, Gruttadauria M, Meo PL, Noto R. Diastereoselective Synthesis of 2-Phenylselenenyl-1,3-anti-Diols and 2-Phenylselenenyl-1,3-anti-Azido-Alcohols via Hydroxyand Azido-Selenenylation Reactions. Molecules. 2005; 10(2):383-393. https://doi.org/10.3390/10020383
Chicago/Turabian StyleRiela, Serena, Carmela Aprile, Michelangelo Gruttadauria, Paolo Lo Meo, and Renato Noto. 2005. "Diastereoselective Synthesis of 2-Phenylselenenyl-1,3-anti-Diols and 2-Phenylselenenyl-1,3-anti-Azido-Alcohols via Hydroxyand Azido-Selenenylation Reactions" Molecules 10, no. 2: 383-393. https://doi.org/10.3390/10020383




