1H- and 13C-NMR Analysis of a Series of 1,2-Diaryl-1H-4,5-dihydroimidazoles
Abstract
:Introduction
| 1 | C6H5 | C6H5 |
| 2 | 4-CH3C6H4 | C6H5 |
| 3 | 4-CH3OC6H4 | C6H5 |
| 4 | 4-ClC6H4 | C6H5 |
| 5 | 4-NO2C6H4 | C6H5 |
| 6 | 3,4-(CH3O)2C6H3 | C6H5 |
| 7 | C6H5 | 4-CH3OC6H4 |
| 8 | C6H5 | 4-ClC6H4 |
| 9 | C6H5 | 3-NO2C6H4 |
| 10 | 4-NO2C6H4 | 4-NO2C6H4 |
| 11 | 2-NO2C6H4 | 4-NO2C6H4 |
| 12 | CH3 | C6H5 |
| 13 | iso-C3H7 | C6H5 |
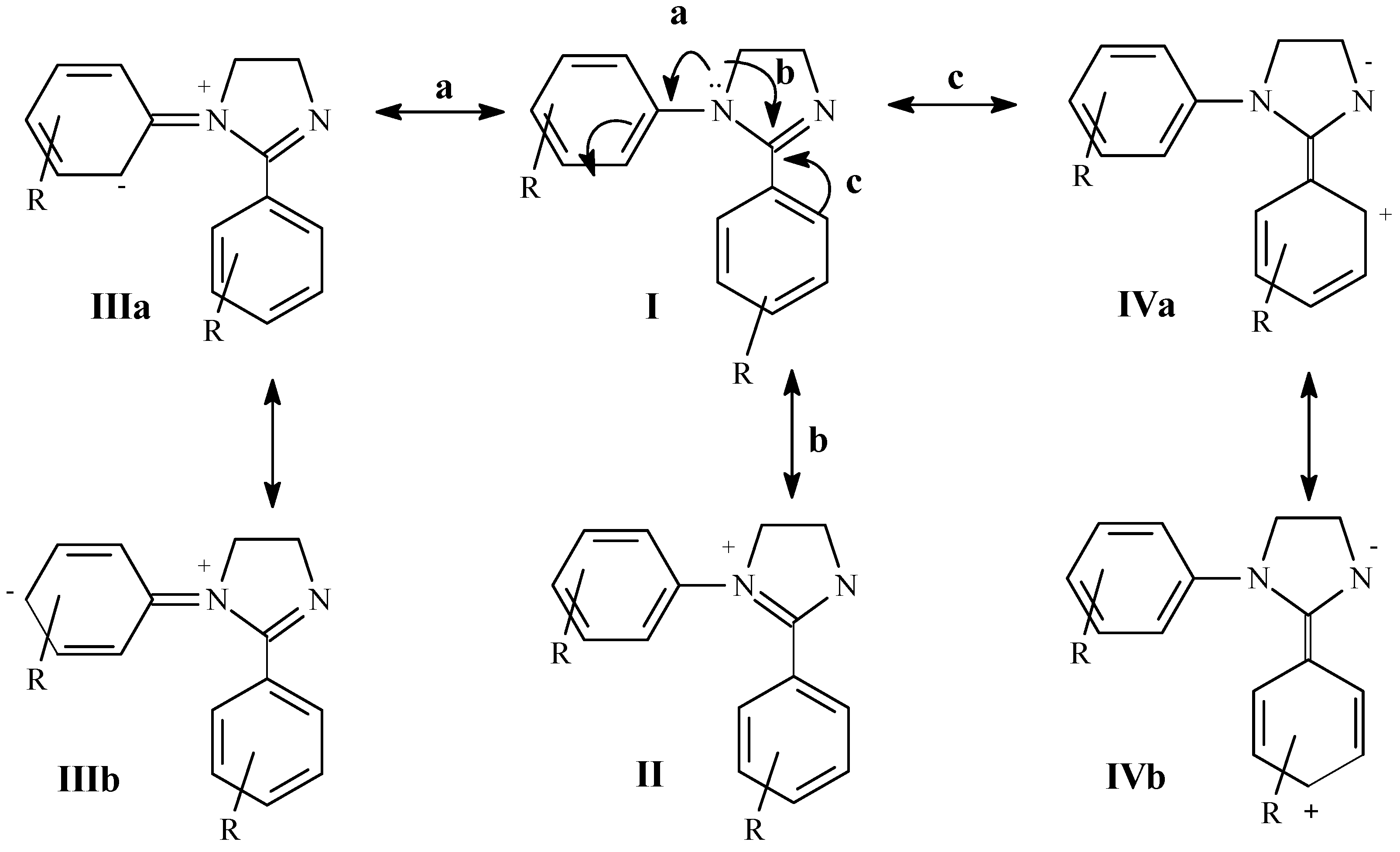
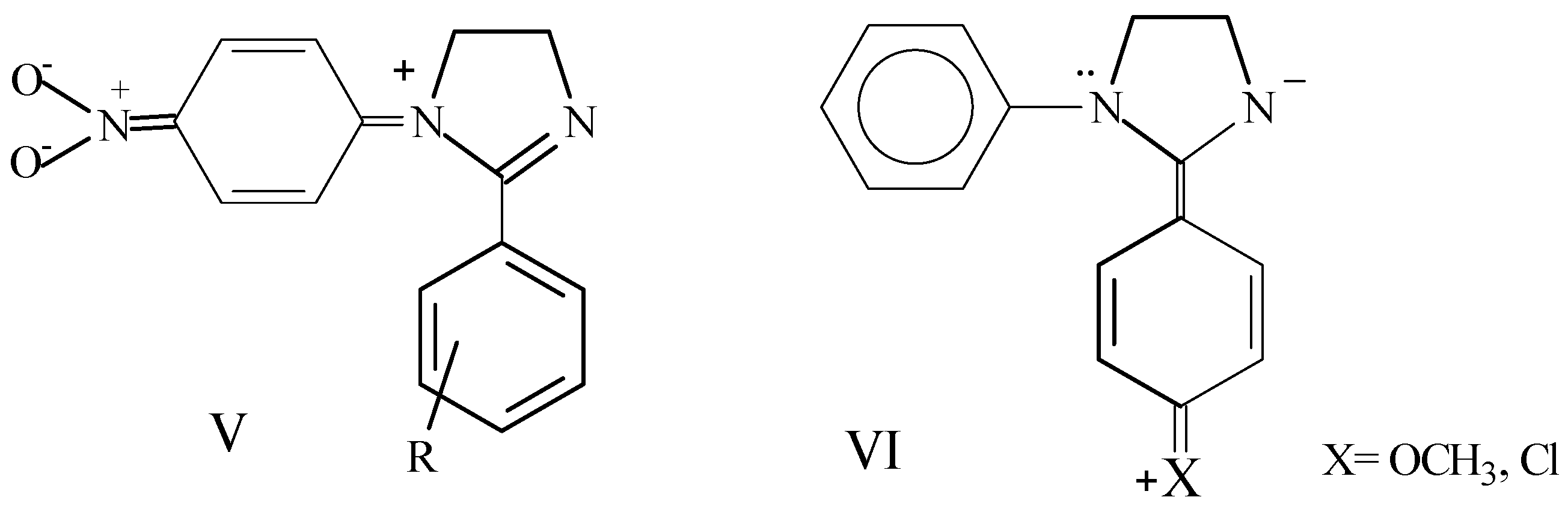
Results and Discussion
| 1 | C6H5 | C6H5 | 4.03 s | 6H: 6.70, dd, J1=8.70, J2=1.01 8H: 6.96, t, J1=7.457H: 7.10, t, J1= 8.50 | 11H,12H: 7.20-7.35, m 10H: 7.45, d, J=7.02 | – | |
| 2 | 4-CH3C6H4 | C6H5 | 4.00 s | 6H: 6.80, d, J=8.20 7H: 7.05, d, J= 8.20 | 11H,12H: 7.32-7.40, m 10H: 7.52, d, J=7.07 | 3.20, s, CH3 | |
| 3 | 4-CH3OC6H4 | C6H5 | 4.00 s | 6H: 6.75, dd, J1=8.2, J2=2.20 7H: 7.00, dd, J1=8.2, J2=2.20 | 11H,12H: 7.20-7.33, m, 10H: 7.45 dd, J1=8.47, J2=1.72 | 3.75, s, CH3 | |
| 4 | 4-ClC6H4 | C6H5 | 4.01 s | 6H: 6.65, dd, J1=6.67, J2=2.21 7H: 7.08, dd, J1=6.67, J2=2.21 | 11H,12H: 7.21-7.34, m 10H: 7.42, dd, J1=6.90, J2=1.54 | – | |
| 5 | 4-NO2C6H4 | C6H5 | 4.15 m [a] | 6H: 6.67, dd, J1=7.02, J2=2.20 7H: 8.05, dd, J1=7.02, J2=2.20 | 10H-12H:7.47-7.51, m | – | |
| 6 |  | C6H5 | 4.00 m [a] | 6H: 6.31, d, J= 2.40 6´H: 6.44, dd, J1=8.40, J2=2.40 7´H: 6.90, d, J=8.40 | 11H,12H: 7.20-7.33, m 10H: 7.46, dd, J1=8.50, J2=1.80 | 3.52, s, OCH3 3.80, s, OCH3 | |
| 7 | C6H5 | 4-CH3OC6H4 | 3.90 s | 6H: 6.78, m [b] 8H: 6.93-6.98, m 7H: 7.15, dd, J1=7.50, J2=2.10 | 11H: 6.78, m [b] 10H: 7.41, d, J=8.90 | 3.65, s, OCH3 | |
| 8 | C6H5 | 4-ClC6H4 | 3.98 s | 6H: 6.74, dd, J1=8.50, J2=1.10 8H: 6.97, td, J1=7.50, J2=1.10 7H: 7.15, dd, J1=8.50, J2=7.50 | 11H: 7.20, d, J=8.50 10H: 7.39, d, J=8.50 | – | |
| 9 | C6H5 |  | 4.08 s | 6H: 6.80, dd, J1=8.30, J2=1.3 8H: 7.02, t, J=7.80 7H: 7.18, dd, J1= 8.30, J2=7.80 | 11´H: 7.43, t, J=7.90 10´H: 7.75, td, J1=6.40, J2=1.50 12H: 8.12, td, J1=7.70, J2=1.50 10H: 8.40, t, J1=1.80 | – | |
| 10 | 4-NO2C6H4 | 4-NO2C6H4 | 4.20 s | 6H: 6.71, d, J=9.01 7H: 8.09, d, J=9.01 | 10H: 7.70, d, J=8.80 11H: 8.25, d, J= 8.80 | – | |
| 11 [c] |  | 4-NO2C6H4 | 4H:4.00 t, J=9.20 3H:4.20 t, J=9.20 | 6´H: 7.08, dd, J1 = 7.90 8H: 7.29, dt, J1 = 7.78, J2 = 1.20 7´H:7.42, dt, J1 = 7.78, J2 = 1.15 7H: 7.84, dd, J1=8.09, J2=1.15 | 10H: 7.61, d, J= 9.09 11H: 8.10, d, J=9.09 | – | |
| 12 | CH3 | C6H5 | 3.80 m [a] | 3.01, s, CH3 | 10H-12H: 7.30, s | – | |
| 13 | CH(CH3)2 | C6H5 | 3.50 m [a] | CH3 :1.00, d CH:2.30, m | 7.30, s | – |
| 1 | C6H5 | C6H5 | 161.7 | 52.5, 53.9 | 5C: 142.1; 6C: 122.5; 7C,10C,11C: 128.6, 128.7, 128.0; 8C: 123.4; 9C: 130.7; 12C: 129.9 | – |
| 2 | 4-CH3C6H4 | C6H5 | 162.0 | 52.7, 53.9 | 5C: 142.0; 6C: 123.0; 7C,10C,11C: 128.2, 128.9, 129.1; 8C: 131.0; 9C: 130.4; 12C: 128.5 | CH3: 20.5 |
| 3 | 4-CH3OC6H4 | C6H5 | 163.2 | 54.8, 55.1 | 5C: 136.1; 6C: 124.9; 7C: 113.8; 8C: 156.1; 9C: 130.9; 10C,11C: 127.8, 128.4; 12C: 129.4 | – |
| 4 | 4-ClC6H4 | C6H5 | 162.1 | 52.8, 53.1 | 5C: 141; 6C: 123.5; 7C,10C,11C: 128.7, 128.5, 128.4; 8C: 128.4; 9C: 130.7; 12C: 130.1 | – |
| 5 | 4-NO2C6H4 | C6H5 | 160.1 | 52.2, 52.9 | 5C: 147.4; 6C: 119.3; 7C: 124.2; 8C: 140.9; 9C: 131.4; 10C,11C: 128.0, 128.3; 12C: 131.4 | – |
| 6 |  | C6H5 | 163.2 | 53.0, 54.1 [a] | 5C: 136.4; 6: 108.2; 6´C,7´C: 111.3, 115.1; 7C: 149.2; 8C: 145.4; 9C: 131.1; 10C,11C: 129.1, 129.2; 12C: 130.1 | CH3O: 55,2, 56.0 [a] |
| 7 | C6H5 | 4-CH3OC6H4 | 162.3 [b] | 52.6, 54.0 | 5C: 143.6; 6C: 122.5; 7C: 128.3; 8C: 123.2; 9C: 123.6, 10C: 130.2; 11C: 113.3; 12C: 160.7 [b] | CH3O: 55,1 |
| 8 | C6H5 | 4-ClC6H4 | 161.4 | 52.7, 53.9 | 5C: 142.6; 6C: 122.5, 7C,11C: 128.1, 128.6; 8C: 123.5; 9C: 129.7; 10C: 129.8; 12C: 135.6 | – |
| 9 | C6H5 |  | 160.1 | 53.3, 54.4 | 5C: 142.4; 6C: 123.1; 7C: 129.1; 8C,10C,12C: 123.7, 124.4, 124.6; 9C: 132.8; 10´C: 134.3; 11C: 147.8; 11´C: 129.3 | – |
| 10 | 4-NO2C6H4 | 4-NO2C6H4 | 160.4 | 53.4, 54.9 | 6C,7C,10C, 11C: 121.9, 126.2, 127.2, 130.7; 9C: 137.9; 5C,8C,12C: 143.3, 147.3, 151.1 | – |
 to the heteroatom [21]. Carbons 4 and 5 of the 4,5-dihydroimidazole system (C3 and C4 respectively in Table 3) appear between 52.2 and 55.8 ppm and are affected in a similar way as C2 by the different substitution on aryl groups.
to the heteroatom [21]. Carbons 4 and 5 of the 4,5-dihydroimidazole system (C3 and C4 respectively in Table 3) appear between 52.2 and 55.8 ppm and are affected in a similar way as C2 by the different substitution on aryl groups.Conclusions
Experimental
General
Acknowledgments
References and Notes
- Faust, J. A.; Yee, L. S. J. Org. Chem. 1961, 26, 4044. [CrossRef]
- Ishikawa, F. Chem. Pharm. Bull. 1980, 28, 1394. [CrossRef]
- McFarland, J. W.; Conover, L. M.; Howes, H. L.; Lynch, J. E.; Chisholm, D. R.; Austin, W. C.; Cornwell, R. L.; Danilewicz, J. C.; Courtney, W.; Morgan, D. H. J. Med. Chem. 1969, 12, 1066.
- Tanabe Seiyaku Co. Ltd. Jpn. Kokai Tokkyo Koho JP 60 51,176, 1985. [Chem. Abstr. 1985, 103, 141951t].
- Le Bian, G.; Rondu, F.; Pelé-Tounian, A.; Wang, X.; Lidy, S.; Toubout, E.; Lamouri, A.; Dive, G.; Huet, J.; Pfeiffer, B.; Renard, P.; Guardiola-Lemaitre, B.; Manéchez, D.; Penicaud, L.; Ktorza, A.; Godfroid, J.-J. J. Med. Chem. 1999, 42, 1587, and references therein.
- Szabo, B. Pharmacol. Therapeut. 2002, 93, 1, and references therein.
- Martin, P.K.; Matthews, H. R.; Rapaport, H.; Thyagarajan, G. J. Org. Chem. 1968, 33, 3758.
- Hughey, J. L.; Knapp, S.; Schugar, H. Synthesis 1980, 9, 489.
- Klem, R. E.; Skinner, H. F.; Walba, H.; Isensee, R. H. J. Heterocycl. Chem. 1970, 7, 403.
- Matsuura, T.; Ito, Y.; Saito, I. Bull. Chem. Soc. Jpn. 1973, 46, 3805. [CrossRef]
- Jones, R. C. F.; Howard, K. J.; Snaith, J. S. Tetrahedron 1997, 53, 1111.
- Salerno, A.; Ceriani, V.; Perillo, I. A. J. Heterocycl. Chem. 1992, 29, 1725, and references therein.
- Salerno, A.; Ceriani, V.; Perillo, I. A. J. Heterocycl. Chem. 1997, 34, 709, and references therein.
- Fernández, B. M.; Reverdito, A. M.; Paolucci, G. A.; Perillo, I. A. J. Heterocycl. Chem. 1987, 24, 1717.
- Anderson, H. W.; Jones, R. C. F.; Saunders, J. J. Chem. Soc. Perkin Trans. 1 1986, 1995, and references therein.
- Pandit, U. K. Pure Appl. Chem. 1994, 66, 759, and references therein.
- Perillo, I.; Lamdan, S. J. Heterocycl. Chem. 1970, 7, 791.
- Fernandez, B.; Perillo, I.; Lamdan, S. J. Heterocycl. Chem. 1973, 10, 915.
- Perillo, I. A.; Caterina, M. C.; Lopez, J; Salerno, A. Synthesis 2004, 6, 851. [CrossRef]
- This feature is also evidence in the reduced basicity of the compounds [18]
- Perillo, I. A.; Buldain, G.; Salerno, A. Heterocycles 2003, 60, 2013, and references therein.
- Cook, M. J.; Katritzky, A. R.; Page, A.D.; Tack, R. D.; Witek, H. Tetrahedron 1976, 32, 1773. [CrossRef]
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Salerno, A.; Perillo, I.A. 1H- and 13C-NMR Analysis of a Series of 1,2-Diaryl-1H-4,5-dihydroimidazoles. Molecules 2005, 10, 435-443. https://doi.org/10.3390/10020435
Salerno A, Perillo IA. 1H- and 13C-NMR Analysis of a Series of 1,2-Diaryl-1H-4,5-dihydroimidazoles. Molecules. 2005; 10(2):435-443. https://doi.org/10.3390/10020435
Chicago/Turabian StyleSalerno, Alejandra, and Isabel A. Perillo. 2005. "1H- and 13C-NMR Analysis of a Series of 1,2-Diaryl-1H-4,5-dihydroimidazoles" Molecules 10, no. 2: 435-443. https://doi.org/10.3390/10020435







