A New iso-Amyl Benzothiazolyl Sulfoxide as an Extractant for Palladium and the Crystal Structure of its Palladium (II) Complex
Abstract
:Introduction
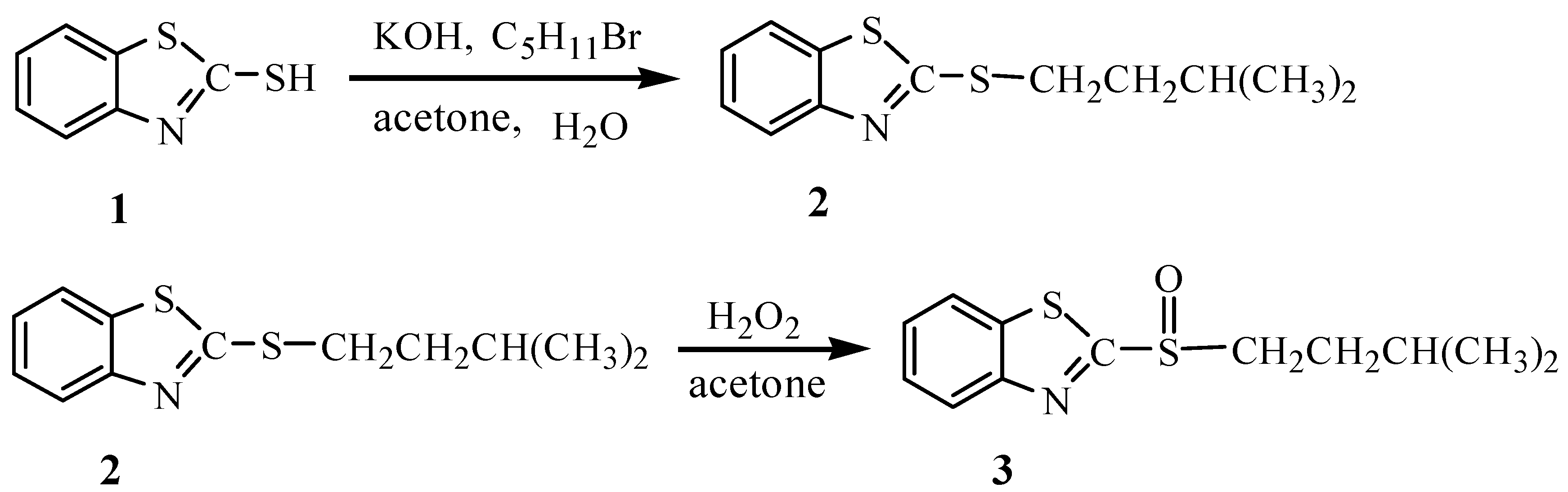
Results and Discussion
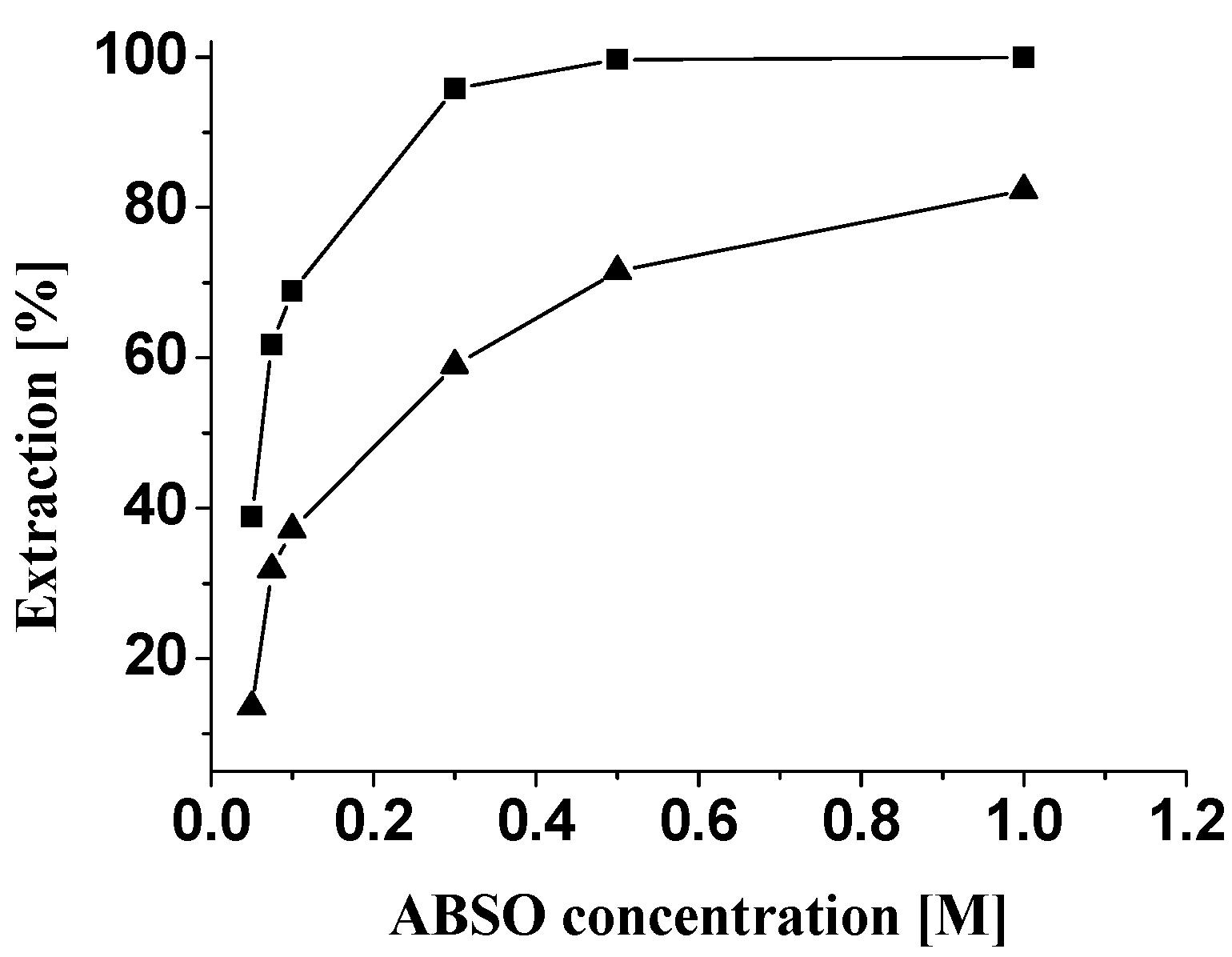
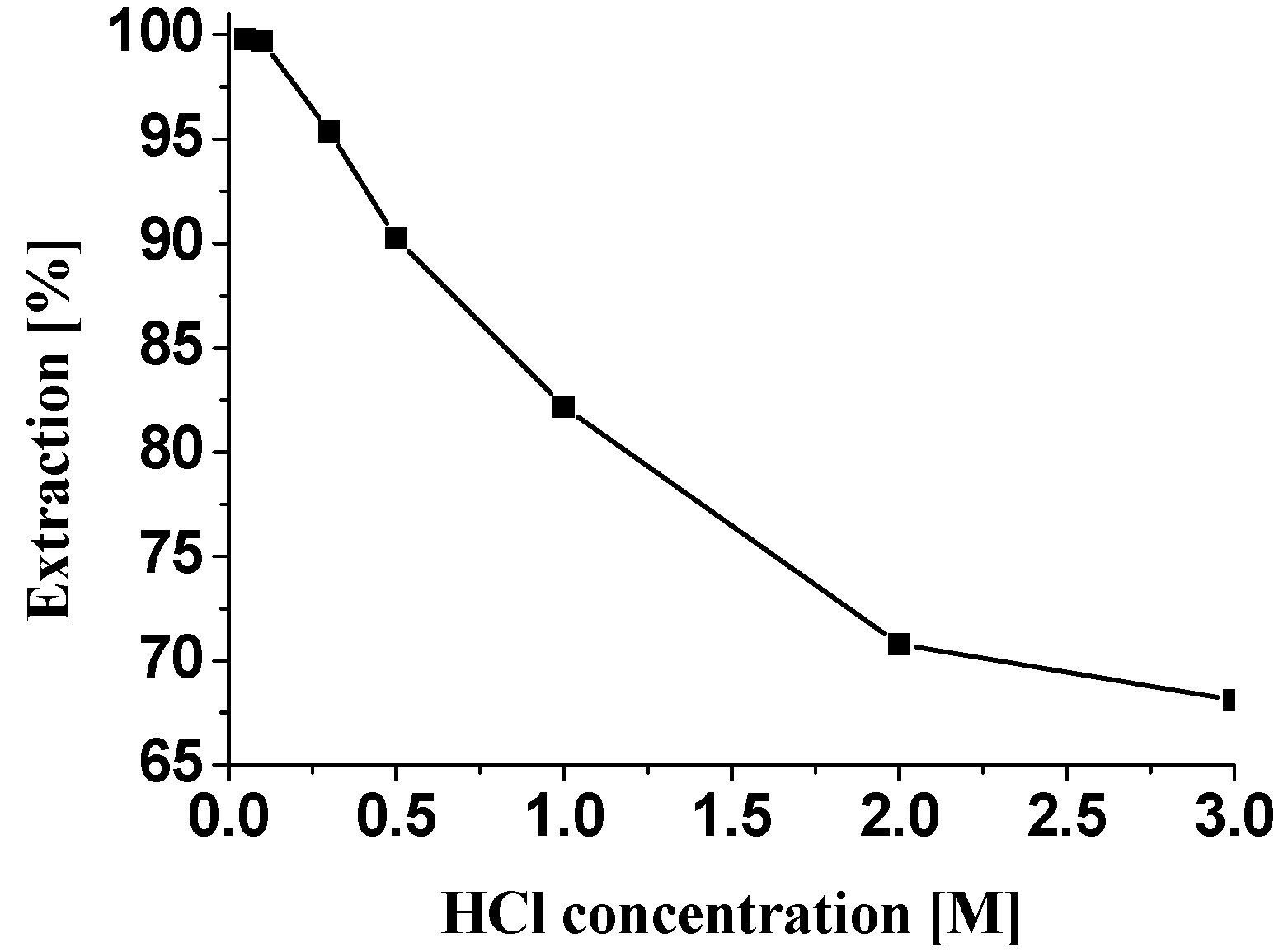
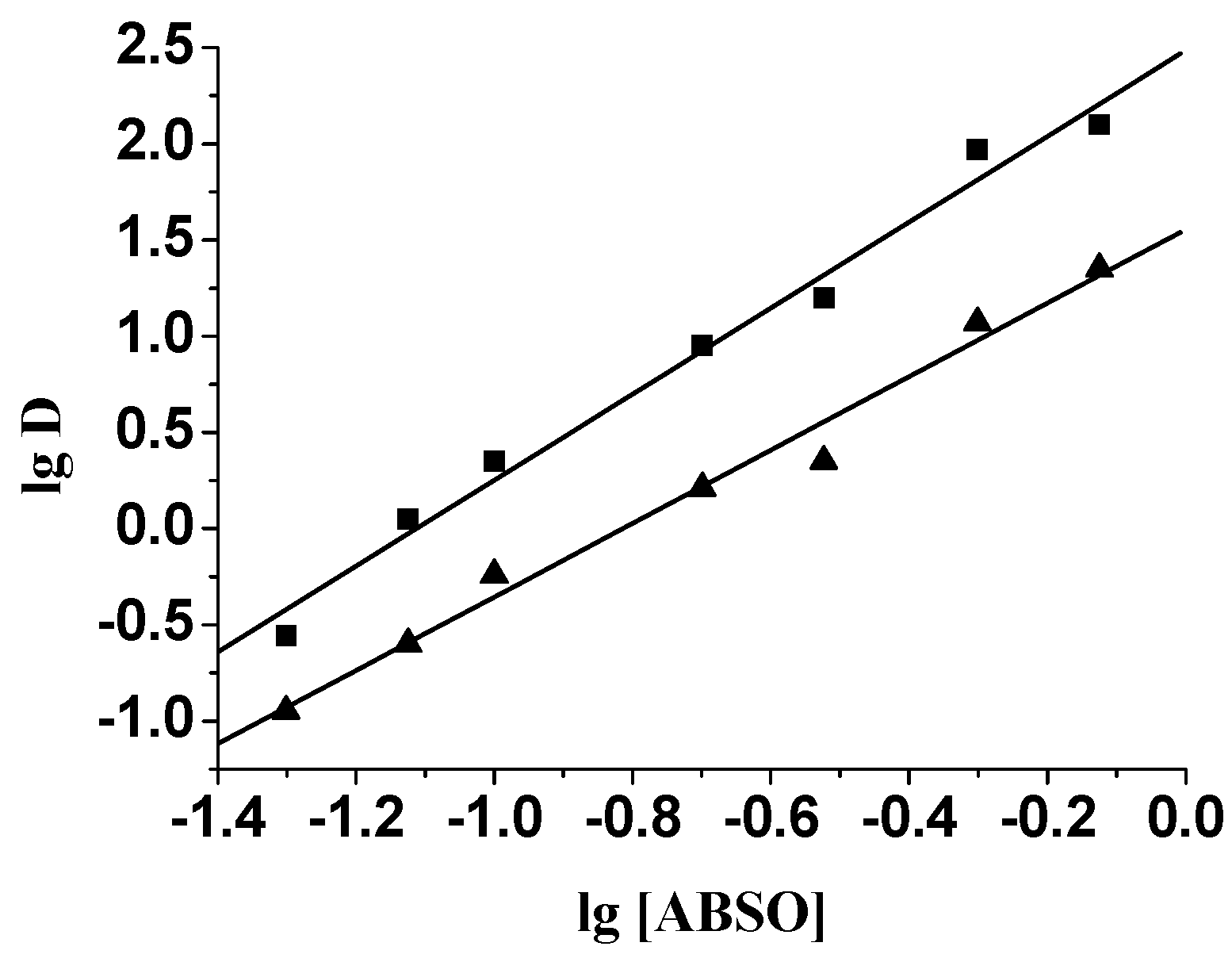
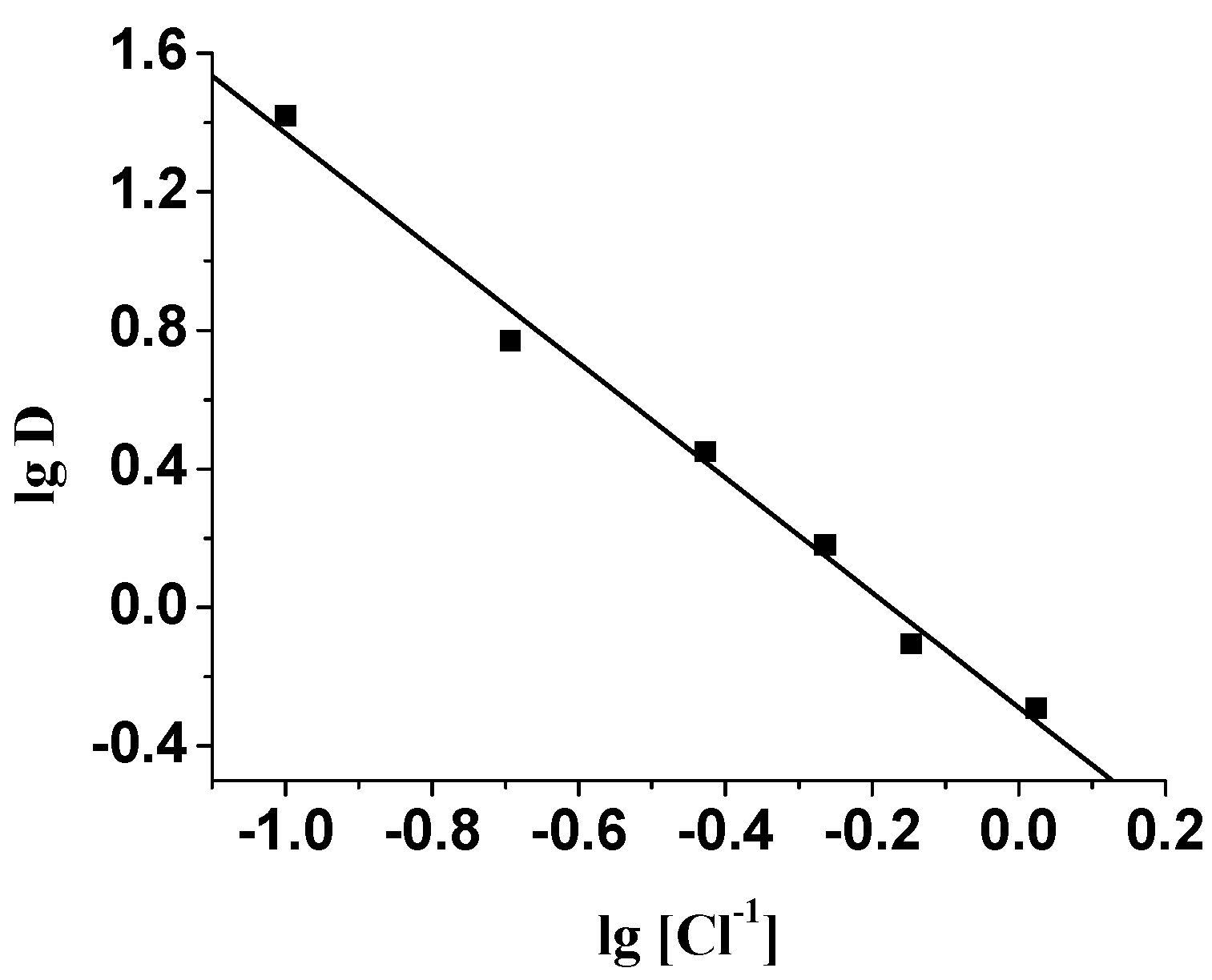
Extraction behavior of ABSO towards Pd(II)
| Stripping Agents | % Recovery | ||||
|---|---|---|---|---|---|
| 0.5 M | 1 M | 2 M | 3 M | 5 M | |
| HCl | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| HNO3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| NaOH | 35.8 | 32.5 | 31.4 | 30.7 | 27.3 |
| NH3·H2O | 97.6 | 99.2 | 99.5 | 99.6 | 99.6 |
| Na2SO3 | 12.4 | 10.6 | 9.8 | 7.5 | 4.4 |
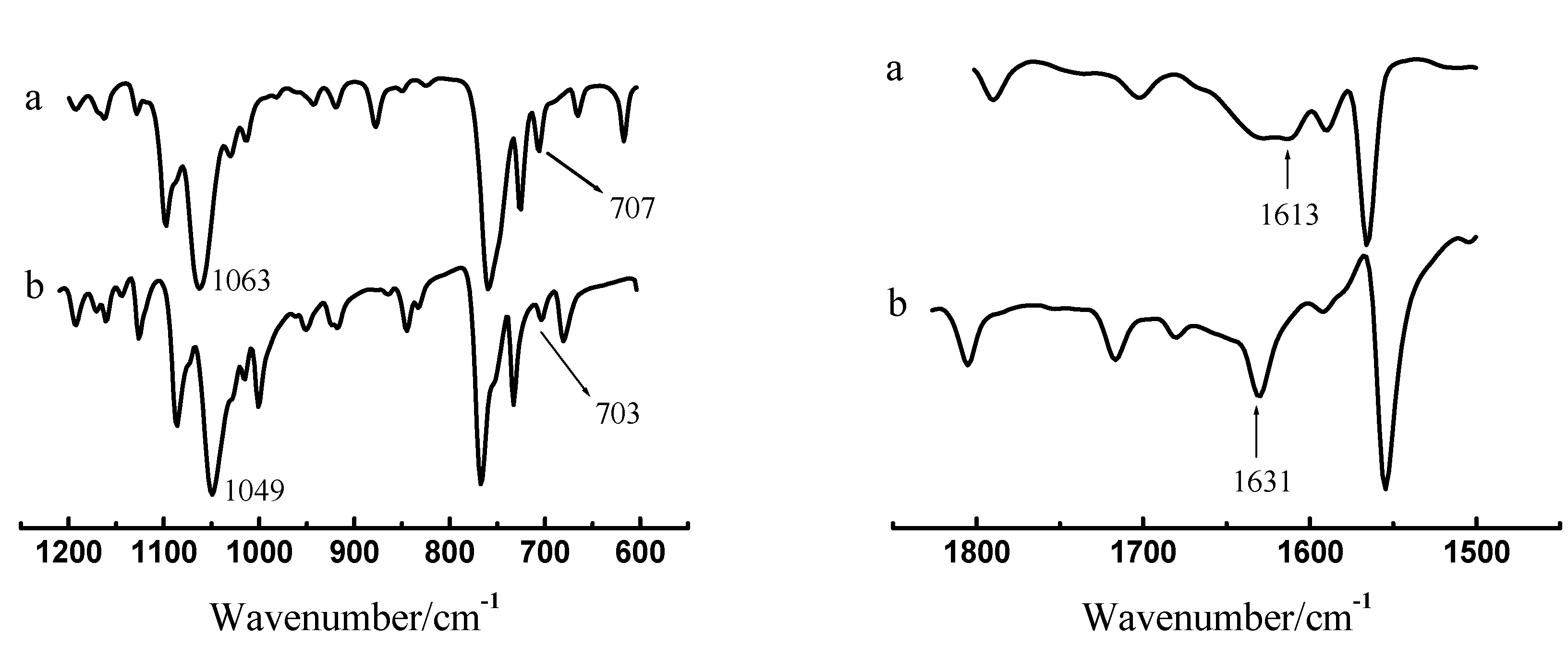
IR spectra of extracted Pd(II)-ABSO adduct
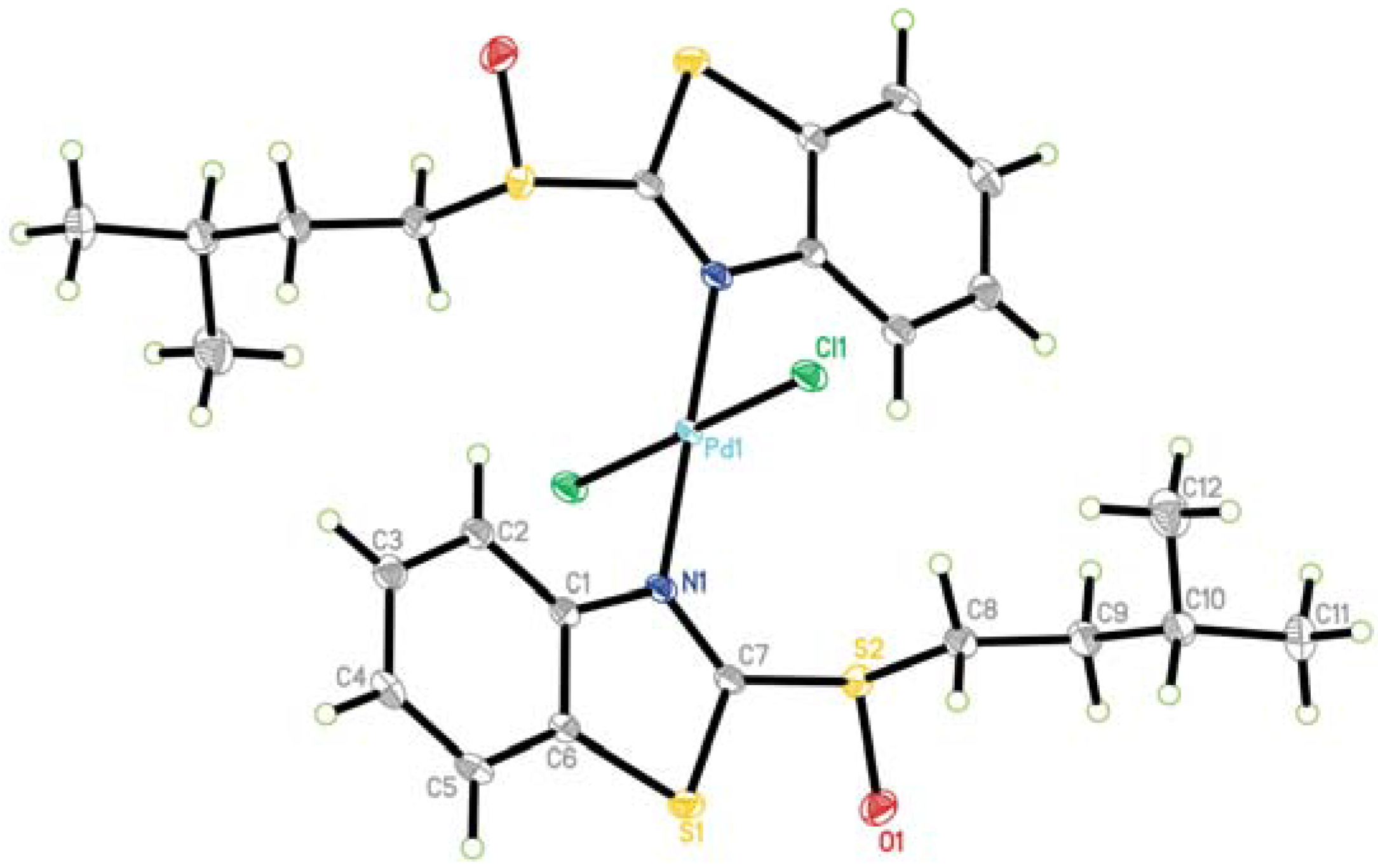
X-Ray Crystallography of PdCl2(ABSO)2 [33]
| Empirical formula | C26H32Cl8N2O2PdS4 | Density (calculated) | 1.658 g/mL |
| Formula weight | 922.78 | Absorption coefficient | 1.334 mm-1 |
| Temperature | 100(2) K | F(000) | 928 |
| Wavelength | 0.71073 Å | Crystal size | 0.25 x 0.08 x 0.08 mm3 |
| Crystal system | Monoclinic | θ range for data collection | 2.53 to 28.29° |
| Space group | P2(1)/c | Reflections collected | 13380 |
| Unit cell dimensions | a = 18.0079(15) Å, α= 90° | Independent reflections | 4358 [R(int) = 0.0367] |
| b = 6.0692(5) Å, β= 116.5450(10)° | Final R indices [I>2sigma(I)] | R1 = 0.0364, wR2 = 0.0811 | |
| c = 18.9080(16) Å, γ= 90° | Goodness-of-fit on F2 | 1.051 | |
| Volume | 1848.7(3) Å3 | R indices (all data) | R1 = 0.0494, wR2 = 0.0862 |
| Z | 2 | Largest diff. peak and hole | 1.350 and -0.811 e.Å-3 |
Conclusions
Experimental
General
Preparation of iso-amyl benzothiazolyl sulfide (2)
Iso-amyl benzothiazolyl sulfoxide (ABSO, 3):
Pd (II) stock solution (1.0 g·L-1):
General extraction procedure
X-ray crystal structure determination
Acknowledgements
References
- Lokhande, T.N.; Anuse, M.A.; Chavan, M.B. Talanta 1998, 46, 163.
- Sahu, R.; Sondhi, S.M.; Gupta, B. Talanta 1995, 42, 401.
- Olesehuk, R.D.; Chow, A. Talanta 1998, 45, 1235.
- Al-bazi, S.J.; Freiser, H. Inorg. Chem. 1989, 28, 417.
- Mathew, V.J.; Khopkar, S.M. Talanta 1997, 44, 1699.
- Baba, Y.; Inoue, K. Ind. Eng. Chem. Res. 1988, 27, 1613. [CrossRef]
- Wisniewski, M. J. Radioanal. Nucl. Chem. 2000, 256, 693. [CrossRef]
- Fujii, T.; Yamana, H.; Watanabe, M.; Moriyama, H. J. Radioanal. Nucl. Chem. 2001, 247, 435. [CrossRef]
- Pearson, R.G. J. Am. Chem. Soc. 1963, 85, 3533.
- Lews, P.A.; Morris, D.F.C.; Short, E.L.; Waters, D.N. J. Less-Common Metals 1976, 45, 193.
- Gu, G.; Cheng, F.; Zhang, Z.; Zeng, F.; Long, T. Solvent Extraction in the Process Industries 1993, 196.
- Rizvi, G.H.; Natarajan, P.R. Fresenius J. Anal. Chem. 1990, 336, 498. [CrossRef]
- Bancroft, D.P.; Cotton, F.A.; Verbruggen, M. Acta Crystallogr. 1989, C45, 1289.
- Langs, D.A.; Hare, C.R.; Little, R.G. Chem. Commun. 1967, 1080.
- Vicente, J.; Arcas, A.; Borrachero, M.V.; Molins, E.; Miravitlles, C. J. Organomet. Chem. 1989, 359, 127.
- Bennett, M.J.; Cotton, F.A.; Weaver, D.L.; Williams, R.J.; Watson, W.H. Acta Crystallogr. 1967, 23, 788.
- Lokhande, T.N.; Anuse, M.A.; Chavan, M.B. Talanta 1998, 46, 163.
- Gholivand, M.B.; Nozari, N. Talanta 2000, 52, 1055.
- He, Z.Q.; Long, T.W.; Gu, G.B. Chemical Metallurgy (in Chinese). 1986, 7, 46.
- Preston, J.S.; du Preez, A. C. Solv. Extr. Ion Exch. 2002, 20, 359. [CrossRef]
- Wu, S.P.; Meng, S.Y.; Gu, G.B. T. Nonferr. Metal Soc. 2004, 14, 202.
- Cotton, F.A.; Francis, R.; Horrocks, W.D. J. Phys. Chem. 1960, 64, 1534.
- Kitching, W.; Moore, C.J.; Doddrell, D. Inorg. Chem. 1970, 9, 541.
- Price, J.H.; Williamson, A.N.; Schramm, R.F.; Wayland, B.B. Inorg. Chem. 1972, 11, 1280.
- Simons, W.W. The Sadtler handbook of infrared spectra; Sadtler Research Laboratories Inc.: Pennsylvania, 1978; p. 341. [Google Scholar]
- Lane, T.J.; Nakagawa, I.; Walter, J.L.; Kandathil, A.J. Inorg. Chem. 1962, 1, 267.
- Furuhashi, A.; Inayoshi, T.; Ouchi, A. Bull. Chem. Soc. Jpn. 1987, 60, 3207. [CrossRef]
- Zhang, L.X.; Zhou, X.G.; Huang, Z.E.; Cai, R.F.; Huang, X.Y. Polyhedron 1999, 18, 1533.
- Julius, G.R.; Cronje, S.; Neveling, A.; Esterhuysen, C.; Raubenheimer, H.G. Helv. Chim. Acta 2002, 85, 3737. [CrossRef]
- West, D.X.; Szczepura, L.F.; Giesen, J.M.; Kaminsky, W.; Kelley, J.; Goldberg, K.I. J. Mol. Struct. 2003, 646, 95.
- Gangopadhyay, J.; Sengupta, S.; Bhattacharyya, S.; Chakraborty, I.; Chakravorty, A. Inorg. Chem. 2002, 41, 2616.
- He, X.F.; Vogels, C.M.; Decken, A.; Westcott, S.A. Polyhedron 2004, 23, 155.
- CCDC 262327 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033; e-mail: deposit@ccdc.cam.ac.uk
- Ito, M.; Furuhashi, A.; Shimoi, M. Polyhedron 1997, 16, 1889.
- Shannon, R.D. Acta Crystallogr. 1976, A32, 751.
- Pauling, L. The Nature of the Chemical Bond, 3rd ed; Cornell Univ. Press: New York, 1960; p. 246. [Google Scholar]
- Ciriano, M.A.; Sebastian, S.; Oro, L.A.; et al. Angew. Chem. 1988, 100, 406.
- Calligaris, M.; Carugo, O. Coord. Chem. Rev. 1996, 153, 83.
- Sample availability: Samples are available from the corresponding author.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Li, Y.-W.; Gu, G.-B.; Liu, H.-Y.; Sung, H.H.Y.; Williams, I.D.; Chang, C.-K. A New iso-Amyl Benzothiazolyl Sulfoxide as an Extractant for Palladium and the Crystal Structure of its Palladium (II) Complex. Molecules 2005, 10, 912-921. https://doi.org/10.3390/10080912
Li Y-W, Gu G-B, Liu H-Y, Sung HHY, Williams ID, Chang C-K. A New iso-Amyl Benzothiazolyl Sulfoxide as an Extractant for Palladium and the Crystal Structure of its Palladium (II) Complex. Molecules. 2005; 10(8):912-921. https://doi.org/10.3390/10080912
Chicago/Turabian StyleLi, Yao-Wei, Guo-Bang Gu, Hai-Yang Liu, Herman H. Y. Sung, Ian D. Williams, and Chi-K. Chang. 2005. "A New iso-Amyl Benzothiazolyl Sulfoxide as an Extractant for Palladium and the Crystal Structure of its Palladium (II) Complex" Molecules 10, no. 8: 912-921. https://doi.org/10.3390/10080912
APA StyleLi, Y.-W., Gu, G.-B., Liu, H.-Y., Sung, H. H. Y., Williams, I. D., & Chang, C.-K. (2005). A New iso-Amyl Benzothiazolyl Sulfoxide as an Extractant for Palladium and the Crystal Structure of its Palladium (II) Complex. Molecules, 10(8), 912-921. https://doi.org/10.3390/10080912






