Investigation of Antioxidant Vitamins (A, E, C) and Lipid Peroxidation Levels in Rats Injected N-(1,3-Benzothiazol-2-yl)-N-(4,5-dihydro-1H-imidazol-2-yl) amine
Abstract
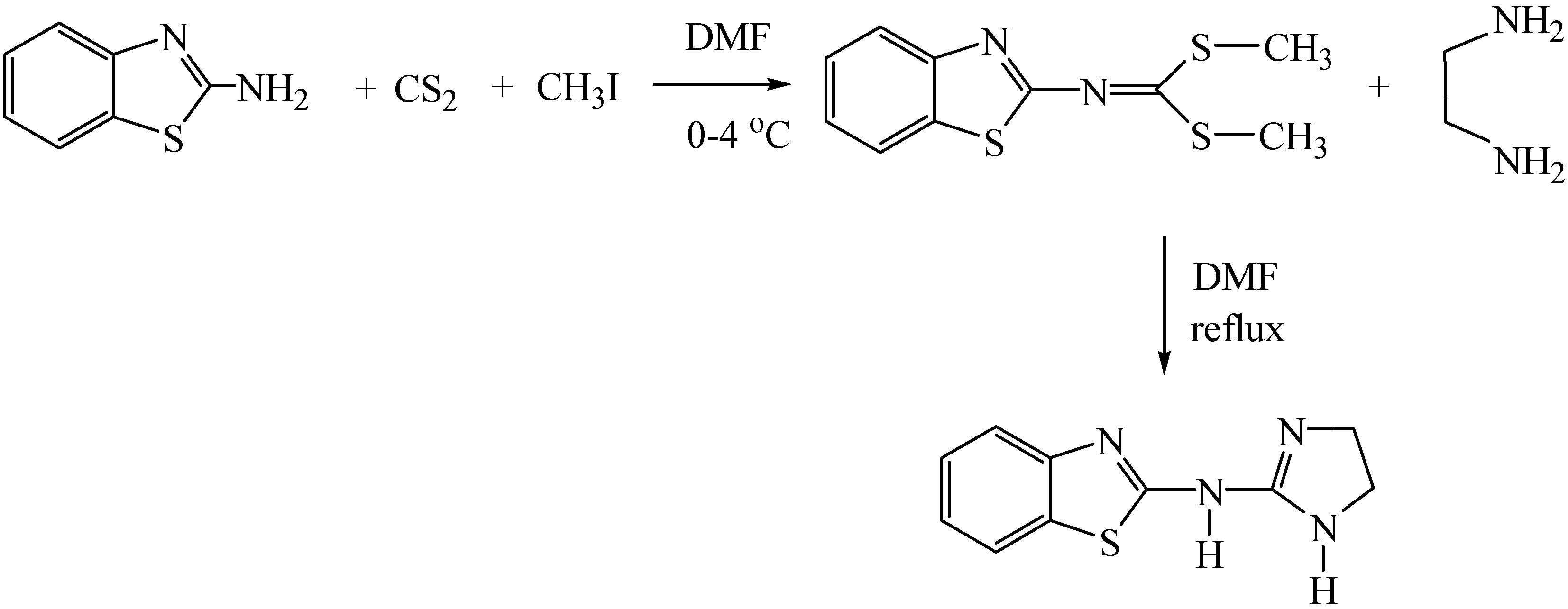
:Introductıon
Results and Discussion
| Vitamin A (µg/mL) | 0.82 ± 0.14 | 0.62 ± 0.17 | < 0.05 |
| Vitamin E (µg/mL) | 8.62 ± 1.30 | 6.55 ± 1.50 | < 0.05 |
| Vitamin C (µg/mL) | 9.64 ± 2.03 | 7.33 ± 1.53 | < 0.05 |
| MDA (nmol/mL) | 1.84 ± 0.37 | 3.99 ± 0.77 | < 0.005 |

Conclusions
Experimental
Animals
Determination of serum Vitamin A and E levels
Determination of serum Vitamin C and MDA levels
Stastical Analysis
References
- Hieble, J.P.; Bondinell, W.E.; Ruffolo, R.R. α- and β-adrenoreceptors: from the gene to the clinic.1. Molecular biology and adrenereceptor subclassification. J. Med. Chem. 1995, 38, 3415–3444. [Google Scholar]
- Ruffolo, R. R.; Bondinell, W.E.; Hielbe, J.P. α- and β-adrenoreceptors: from the gene to the clinic.2. Structure–activity relationships and therepeutic applications. J. Med. Chem. 1995, 38, 3681–3716. [Google Scholar]
- Matsuo, M.; Taniguchi, K.; Katsura, Y.; Kamitani, T.; Ueda, I. New 2-aryliminoimidazolidines. I. Synthesis and antihypertensive properties of 2-(2-phenoxyphenylimino)imidazolidines and related compounds. Chem. Pharm. Bull. (Tokyo). 1985, 33, 4409–4421. [Google Scholar]
- Dardonville, C.; Rozas, I. Imidazoline binding sites and their ligands: An overview of the different chemical structures. Med. Res. Rev. 2004, 24, 639–61. [Google Scholar]
- Mundla, S. R.; Wilson, L. J.; Klopfenstein, S. R.; Seibel, W. L.; Nikolaides, N. N. A novel method for the efficient synthesis of 2-arylamino-2-imidazolines. Tetrahedron Lett. 2000, 41, 6563–6566. [Google Scholar]
- Paterson, L M.; Tyacke, R. J.; Nutt, D. J.; Hudson, A. L. Relationship between imidazoline(2) sites and monoamine oxidase. Ann. N. Y. Acad. Sci. 2003, 1009, 353–356. [Google Scholar]
- Alemany, R.; Olmos, G.; Garcia-Sevilla, J. A. Labelling of I2B-imidazoline receptors by [3H]2-(2-benzofuranyl)-2-imidazoline (2-BFI) in rat brain and liver: characterization, regulation and relation to monoamine oxidase enzymes. Naunyn Schmiedebergs Arch. Pharmacol. 1997, 356, 39–47. [Google Scholar]
- Sanchez-Blazquez, P.; Boronat, M. A.; Olmos, G.; Garcia-Sevilla, J. A.; Garzon, J. Activation of I(2)-imidazoline receptors enhances supraspinal morphine analgesia in mice: a model to detect agonist and antagonist activities at these receptors. Br. J. Pharmacol. 2000, 130, 146–152. [Google Scholar]
- Holsapple, M. P.; Trizzino, J.; Nichols, D. E.; Yim, G. K. Therapeutic and adjunctive applications of an imidazoline anti inflammatory agent. J. Pharmacol. Exp. Ther. 1983, 224, 567–571. [Google Scholar]
- Kiec-Kononowicz, K.; Szymanska, E.; Motyl, M.; Holzer, W.; Bialecka, A.; Kasprowicz, A. Synthesis, spectral and antimicrobial properties of 5-chloroarylidene aromatic derivatives of imidazoline-4-one. Pharmazie 1998, 53, 680–684. [Google Scholar]
- Terasawa, K.; Sugita, Y.; Yokoe, I.; Fujisawa, S.; Sakagami, H. Cytotoxic activity of 5-benzoylimidazole and related compounds against human oral tumor cell lines. Anticancer Res. 2001, 21, 1081–1086. [Google Scholar]
- Olmos, G.; De Gregorio-Rocasolano, N.; Paz Regalado, M.; Gasull, T.; Assumpcio Boronat, M; Trullas, R.; Villarroel, A.; Lerma, J.; Garcia-Sevilla, J. A. Protection by imidazol(ine) drugs and agmatine of glutamate-induced neurotoxicity in cultured cerebellar granule cells through blockade of NMDA receptor. Br. J. Pharmacol. 1999, 127, 1317–1326. [Google Scholar]
- Graf, E.; Sieck, A.; Wenzl, H.; Winkelmann, J. Animal experiments on the safety pharmacology of lofexidine. Arzneimittel-Forsch. 1982, 32, 931–940. [Google Scholar]
- Laila, G.; Yues, A.; Bernard, H.; Claude, J.; Gerard, C.; Gerard, S. Biological variability of superoxide dismutase, glutathione peroxidase and catalase in blood. Clin. Chem. 1991, 37, 1932–1937. [Google Scholar]
- Halliwell, B. Free radical antioxidants in human disease. Curiosity, cause or consequence. Lancet 1994, 344, 721–724. [Google Scholar]
- Kappus, H.A. A survey of chemicals inducing lipid peroxidation in biological systems. Chem. Phys. Lipids 1987, 45, 105–115. [Google Scholar]
- Fang, J.L.; Vaca, C.E.; Valsta, L.M.; Mutanen, M. Determination of DNA adducts of malonaldehyde in humans: effects of dietary fatty acid composition. Carcinogenesis 1996, 17, 1035–1040. [Google Scholar]
- Sarkar, S.; Yadav, P.; Bhatnagar, D.J. Cadmium-induced lipid peroxidation and the antioxidant system in rat erythrocytes: the role of antioxidants. Trace Elem. Med. Biol. 1997, 11, 8–13. [Google Scholar]
- Cheesman, K.H.; Slater, T.F. Introduction to free radical biochemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar]
- Jacob, R.A.; Burri, B.J. Oxidative damage and defense. Am. J. Clin. Nutr. 1996, 63, 985–990. [Google Scholar]
- Can-Eke, B.; Puskullu, M.O.; Buyukbingol, E.; Iscan, M. A study on the antioxidant capacities of some benzimidazoles in rat tissues. Chem. Biol. Interact. 1998, 113, 65–77. [Google Scholar]
- Papaccio, G.; Nicoletti, F.; Pisanti, FA; Galdieri, M.; Bendtzen, K. An imidazoline compound completely counteracts interleukin-1[beta] toxic effects to rat pancreatic islet [beta] cells. Mol Med. 2002, 8, 536–545. [Google Scholar]
- Sogawa, S.; Nihro, Y.; Ueda, H.; Miki, T.; Matsomota, H.; Satoh, T. Protective effects of hydroxychalcones on free radical-induced cell damage. Biol. Pharm. Bull. 1994, 17, 251–256. [Google Scholar]
- Servi, S. The efficient synthesis of 2-arylamino-2-imidazolines, 2-heteroaryl substituted benzimidazoles, and their morpholin-4-ylmethyl derivatives. S. Afr. J. Chem. 2002, 55, 119–123. [Google Scholar]
- Genc, M; Servi, S. Microwave-Assisted Rapid Synthesis of 2-Arylamino-2-Imidazolines on a Solid-Support. Heteroatom Chem. 2005, 16. in press. [Google Scholar]
- Miller, K.W.; Lorr, N.A.; Yang, C.S. Simultaneous determination of plasma retinol, α-tocopherol, lycopene, α-carotene, and β-carotene by high performance liquid- chromatography. Anal. Biochem. 1984, 138, 340–345. [Google Scholar]
- Cerhata, D.; Bauerova, A.; Ginter, B. Determination of ascorbic acid in serum using high performance liquid-chromatography and its correlation with spectrophometric (colorimetric) determination. Caska Slov. Farm. 1994, 43, 166–168. [Google Scholar]
- Tavazzi, B.; Lazzarino, G.; Di-Piero, D.; Giardina, B. Malondialdehyde production and ascorbate decrease are associated to the reperfusion of the isolated postischemic rat heart. Free Radic. Biol. Med. 1992, 13, 75–78. [Google Scholar]
- Karatas, F.; Karatepe, M.; Baysar, A. Determination of free malondialdehyde in human serum by high performance liquid chromatography. Anal. Biochem. 2002, 311, 76–79. [Google Scholar]
- Sample Availability: Contact the authors.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Karatas, F.; Kara, H.; Servi, S.; Tug, T.; Erulas, F.A.; Koca, M. Investigation of Antioxidant Vitamins (A, E, C) and Lipid Peroxidation Levels in Rats Injected N-(1,3-Benzothiazol-2-yl)-N-(4,5-dihydro-1H-imidazol-2-yl) amine. Molecules 2005, 10, 922-928. https://doi.org/10.3390/10080922
Karatas F, Kara H, Servi S, Tug T, Erulas FA, Koca M. Investigation of Antioxidant Vitamins (A, E, C) and Lipid Peroxidation Levels in Rats Injected N-(1,3-Benzothiazol-2-yl)-N-(4,5-dihydro-1H-imidazol-2-yl) amine. Molecules. 2005; 10(8):922-928. https://doi.org/10.3390/10080922
Chicago/Turabian StyleKaratas, F., H. Kara, S. Servi, T. Tug, F. A. Erulas, and M. Koca. 2005. "Investigation of Antioxidant Vitamins (A, E, C) and Lipid Peroxidation Levels in Rats Injected N-(1,3-Benzothiazol-2-yl)-N-(4,5-dihydro-1H-imidazol-2-yl) amine" Molecules 10, no. 8: 922-928. https://doi.org/10.3390/10080922




