Terpenoids from Cleome droserifolia (Forssk.) Del
Abstract
:INTRODUCTION
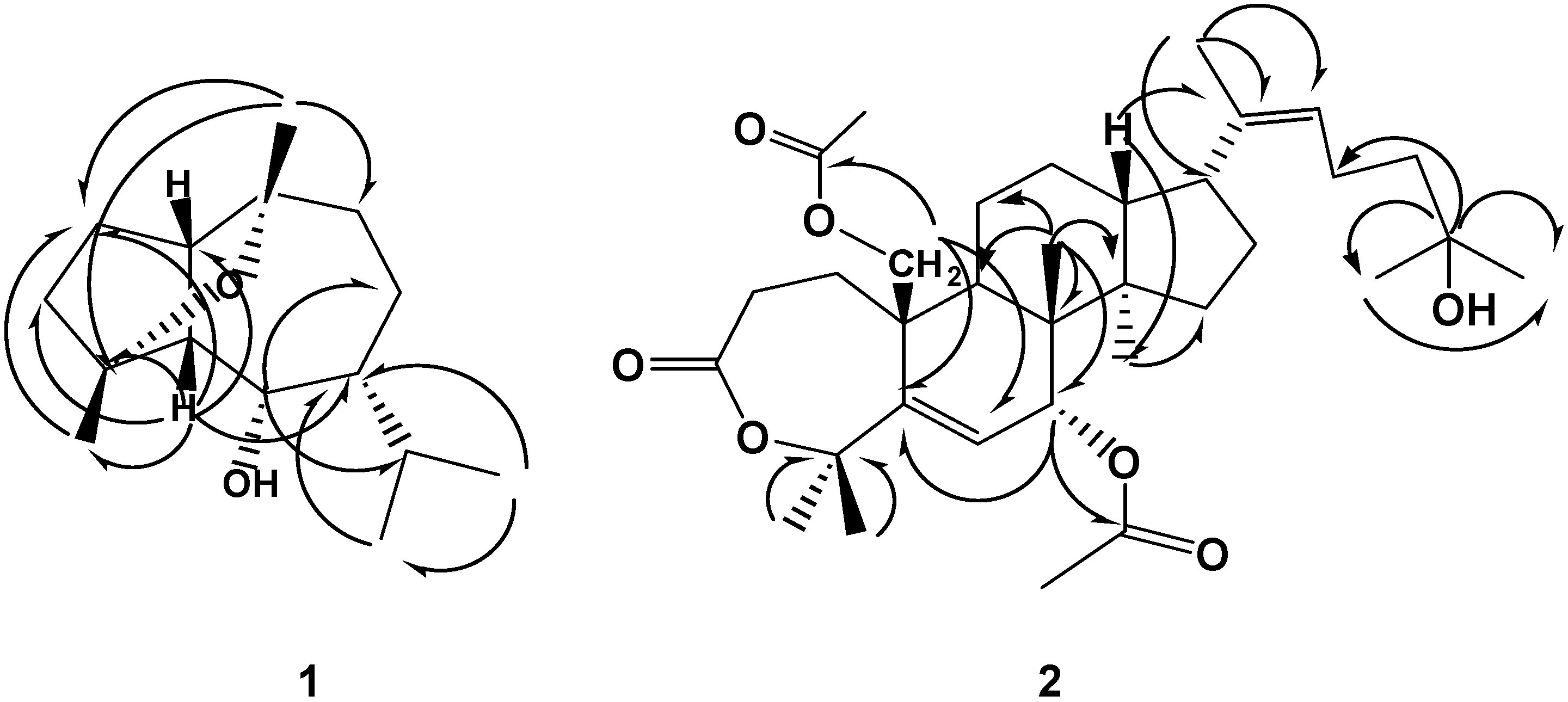
Results and Discussion:

Experimental
General
Plant material
Isolation
| Compound 1 | Compound 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Position No. | δc | δH | Position No. | δc | δH | Position No. | δc | δH | |
| 1 | 53.3 d | 2.34 m | 1 | 34.6 t | 2.05 m 2.46 m | 16 | 29.3 t | 1.36 m 1.65 m | |
Acknowledgments
References
- Täckholm, V. Students` Flora of Egypt, 2nd Ed. ed; Cairo University Press: Cairo, Egypt, 1974; p. 167. [Google Scholar]
- Zohary, M. Flora Palaestina; The Israel Academy of Sciences and Humanities: Jerusalem, 1966; vol. I, p. 245. [Google Scholar]
- Boulos, L. Medicinal Plants of North Africa; Reference Publication Inc.: Michigan, USA, 1983; p. 52. [Google Scholar]
- Boulos, L. Flora of Egypt; Al Hadara Publishing: Cairo, Egypt, 1999; p. 177. [Google Scholar]
- Ghazanfar, S.A. Handbook of Arabian Medicinal Plants; CRC Press: Boca Raton-London-Tokyo, 1994; p. 76. [Google Scholar]
- Chopra, R.N.; Chopra, I.C.; Handa, K.L.; Kapur, L.D. Chopra`s Indigenous Drugs of India; Dhur & Sons, Private Limited: Calcutta, India, 1972; pp. 321, 669. [Google Scholar]
- Yang, S.S.; Mabry, T.J.; El-Fishawy, A.M.; El-Kashoury, E.A.; Abdel-Kawy, M.A.; Soliman, F.M. Flavonoids of Cleome droserifolia (Forssk.) Del. Egypt. J. Pharm. Sci. 1990, 31, 443. [Google Scholar]
- Abdel-Hady, N.M. Pharmacognostical Investigation and Biological Verification of Some Recipes and Preparations of Natural Origin for the Treatment of Diabetes. MS Thesis, Faculty of Pharmacy (Girls), Al-Azhar University, Cairo, 1998. [Google Scholar]
- Yaniv, Z.; Dafni, A.; Friedman, J.; Palevitch, D. Plants used for the treatment of Diabetes in Israel. J. Ethnopharmacol. 1987, 19, 145. [Google Scholar]
- Nocola, W.G.; Ibrahim, K.M.; Mikhail, T.H.; Girgis, R.B.; Khadr, M.E. Role of the hypoglycemic plant extract Cleome droserifolia in improving glucose and lipid metabolism and its relation to insulin resistance in fatty liver. Boll. Chim. Farm. 1996, 135, 507. [Google Scholar]
- Seif El-Din, A.A.; Darwish, F.A.; Abou-Donia, A.A. Flavonoids from Cleome droserifolia (Forssk.) Del. Growing in Egypt. Egypt. J. Pharm. Sci. 1987, 28, 313. [Google Scholar]
- Seif El-Nasr, M.M.; Youssef, M.M.; Helmy, M. Flavonoids of Cleome droserifolia (Forssk.) Del. Fitoterapia 1984, 55, 231. [Google Scholar]
- Abdel-Kawy, M.A.; El-Deib, S.; El-Khyat, Z.; Mikhail, Y.A. Chemical and biological studies of Cleome droserifolia (Forssk.) Del. Part-I. Egypt. J. Biomed. Sci. 2000, 6, 204. [Google Scholar]
- Fushiya, S.; Kishi, Y.; Hattori, K.; Batkhuu, J.; Takano, F.; Singab, A.N.B.; Okuyama, T. Flavonoids from Cleome droserifolia Suppress NO Production in Activated Macrophages in Vitro. Planta Med. 1999, 65, 404. [Google Scholar]
- Diab, L. Pharmacognostical Study of Certain Cleome Species Growing in Egypt. MS Thesis, Faculty of Pharmacy (Boys) Al-Azhar University, Cairo, Egypt, 1992. [Google Scholar]
- Harraz, F.M.; Ulubelen, A.; Osuz, S.; Tan, N. Dammarane triterpenes from Cleome amblyocarpa. Phytochemistry 1995, 39, 175. [Google Scholar]
- Nagaya, H.; Tobita, Y.; Nagae, T.; Itokawa, H.; Takeya, K.; Halim, A.F.; Abdel-Halim, O.B. Cytotoxic triterpenes from Cleome africana. Phytochemistry 1997, 44, 1115. [Google Scholar]
- Tsichritzis, F.; Abdel-Mogip, M.; Jakupovic, J. Dammarane triterpenes from Cleome africana. Phytochemistry 1993, 33, 423. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Qazi, S.; Bin Zia, N.; Xu, C.; Clardy, J. Cleocarpone, A triterpenoid from Cleome brachycarpa. Phytochemistry 1990, 29, 670. [Google Scholar]
- Ahmad, V.U.; Alvi, K.A. Deacetoxybrachycarpone, A trinortriterpenoid from Cleome brachycarpa. Phytochemistry 1987, 26, 315. [Google Scholar]
- Ahmad, V.U.; Alvi, K.A. The Molecular Structure and Absolute Configuration of Brachycarpone, A new trinortriterpenoid dilactone from Cleome brachycarpa. J. Nat. Prod. 1986, 49, 249. [Google Scholar]
- Hussein, N.S.; Ahmed, A.A.; Darwish, F.M. Sesquiterpenes from Cleome droserifolia. Pharmazie 1994, 49, 76. [Google Scholar]
- Jente, R.; Jakupovic, J.; Olatunji, G.A. A Cembranoid Diterpene from Cleome viscosa. Phytochemistry 1990, 29, 666. [Google Scholar]
- Ahmad, VU.; Zahid, M.; Ali, M.S.; Jassbi, A.R.; Abbas, M.; Ali, Z.; Iqbal, M.Z. Bucharioside and buchariol from Salvia bucharica. Phytochemistry 1999, 52, 1319. [Google Scholar]
- Dou, D.; Chen, Y.; Liang, L.; Pang, F.; Shimizu, N.; Takeda, T. Six new dammarane-type trirepene saponins from the leaves of Panax ginseng. Chem. Pharm. Bull. 2001, 49, 442. [Google Scholar]
- El-Askary, H. Pregnene glycoside and monoterpene derivative from Solenostemma argel Hayne. Bull. Fac. Pharm. Cairo Univ. 2003, 41, 131. [Google Scholar]
- Zou, K.; Zhu, S.; Meselhy, R.; Tohda, C.; Cai, S.; Komatsu, K. Dammarane-type saponins from Panax japonicus and their neurite outgrowth activity in SK-N-SH cells. J. Nat. Prod. 2002, 65, 1288. [Google Scholar]
- Nakamura, N.; Kojima, S.; Aura, Y.; Meselhy, R.; Hattori, M.; Gupta, M.; Correa, M. Dammarane-type triterpenes from Cordia spinescens. Phytochemistry 1997, 46, 1139. [Google Scholar] [CrossRef]
- Rouf, A.S.; Ozaki, Y.; Rashid, M.A.; Rui, J. Dammarane derivatives from the dried fruits of Forsythia suspensa. Phytochemistry 2001, 56, 815. [Google Scholar]
- Goad, L.J.; Akihisa, T. Analysis of Sterols; Blackie Academic & Professional: Glasgow, UK, 1997; p. 380. [Google Scholar]
- Sample Availability: Contact the author.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
El-Askary, H.I. Terpenoids from Cleome droserifolia (Forssk.) Del. Molecules 2005, 10, 971-977. https://doi.org/10.3390/10080971
El-Askary HI. Terpenoids from Cleome droserifolia (Forssk.) Del. Molecules. 2005; 10(8):971-977. https://doi.org/10.3390/10080971
Chicago/Turabian StyleEl-Askary, Hesham I. 2005. "Terpenoids from Cleome droserifolia (Forssk.) Del" Molecules 10, no. 8: 971-977. https://doi.org/10.3390/10080971




