The tert-Amino Effect in Heterocyclic Chemistry. Synthesis of Spiro Heterocycles
Abstract
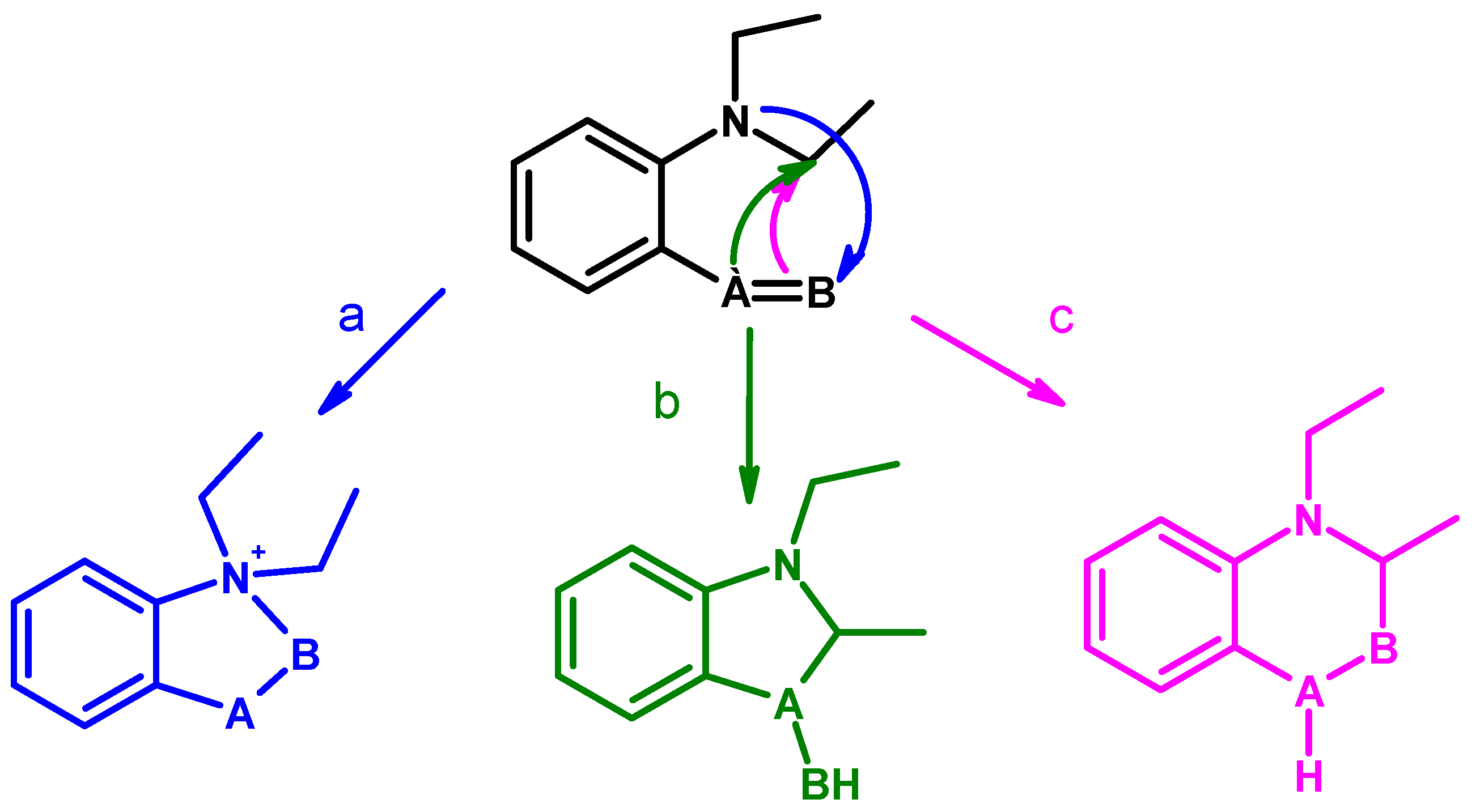
:
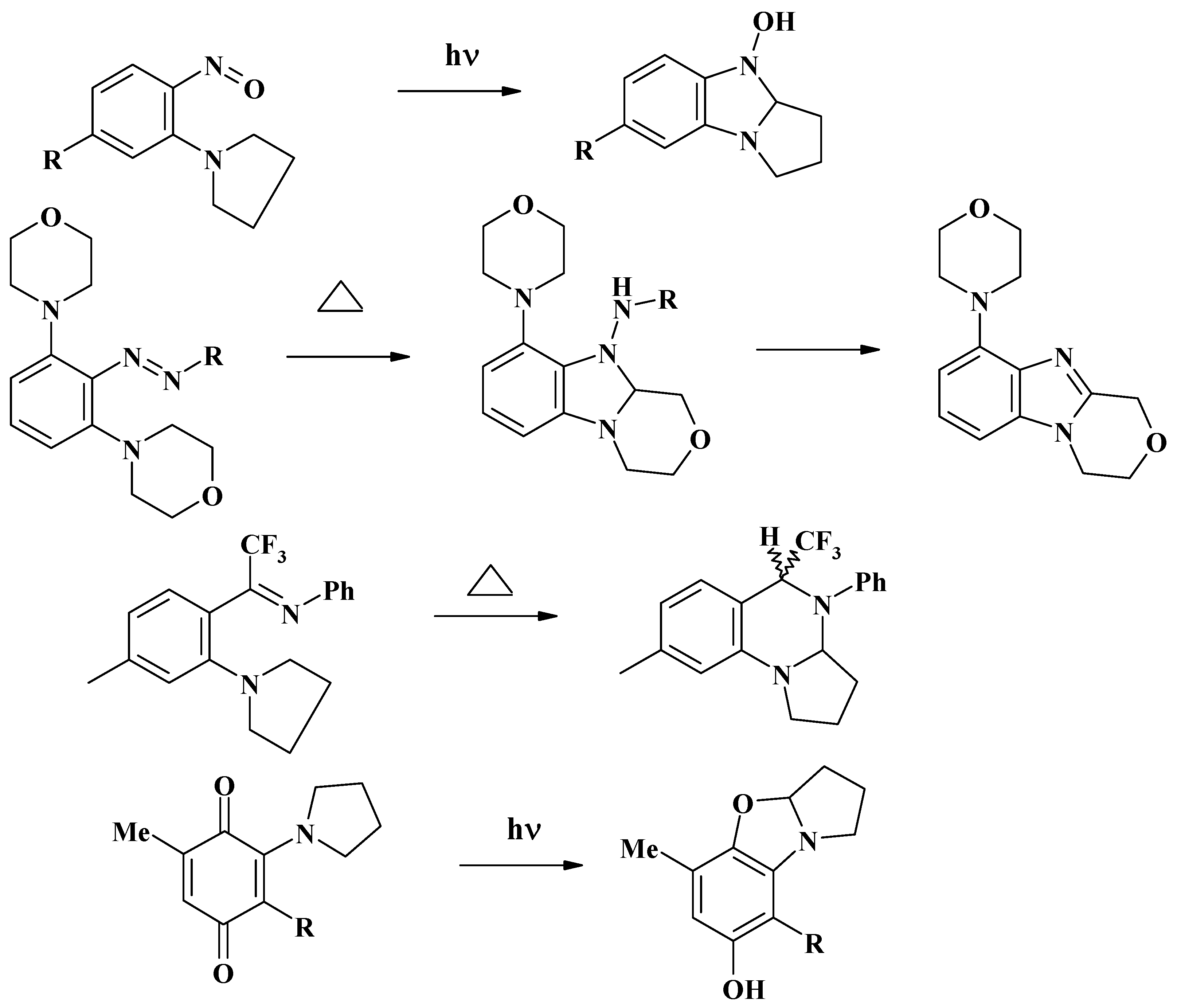
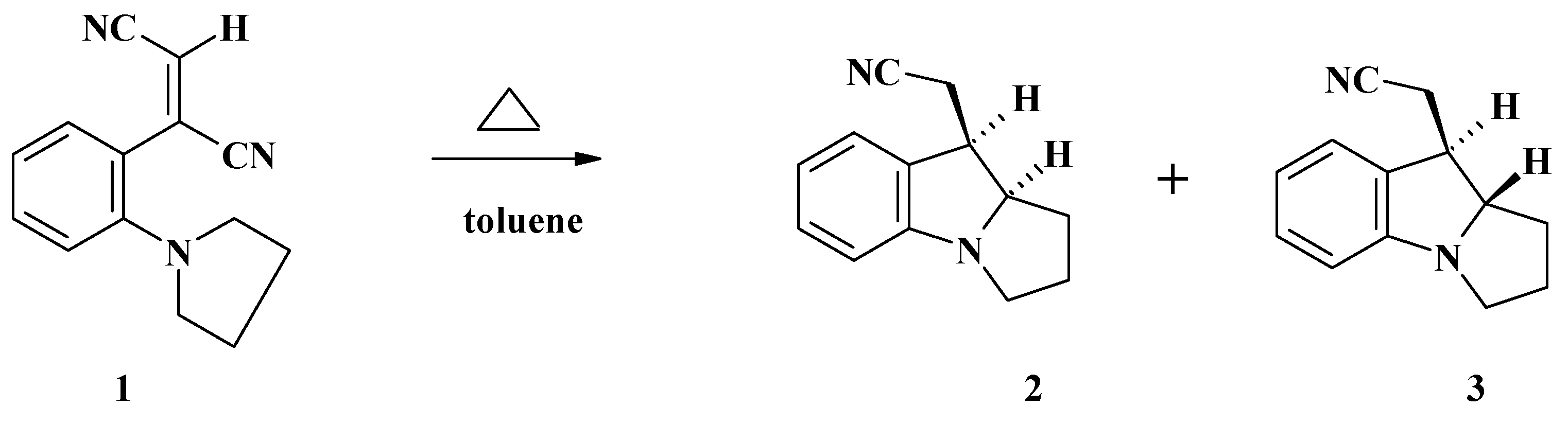
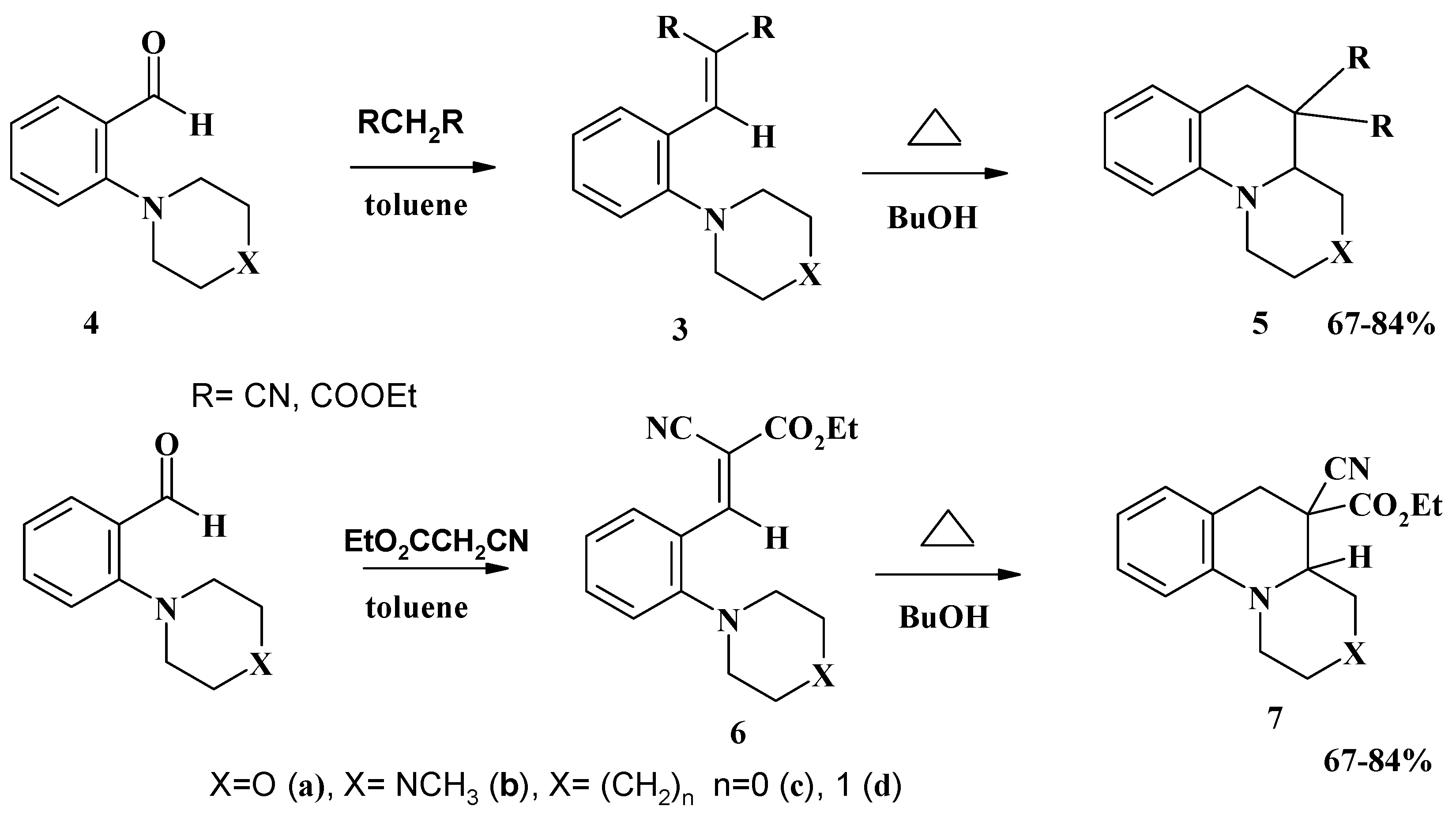
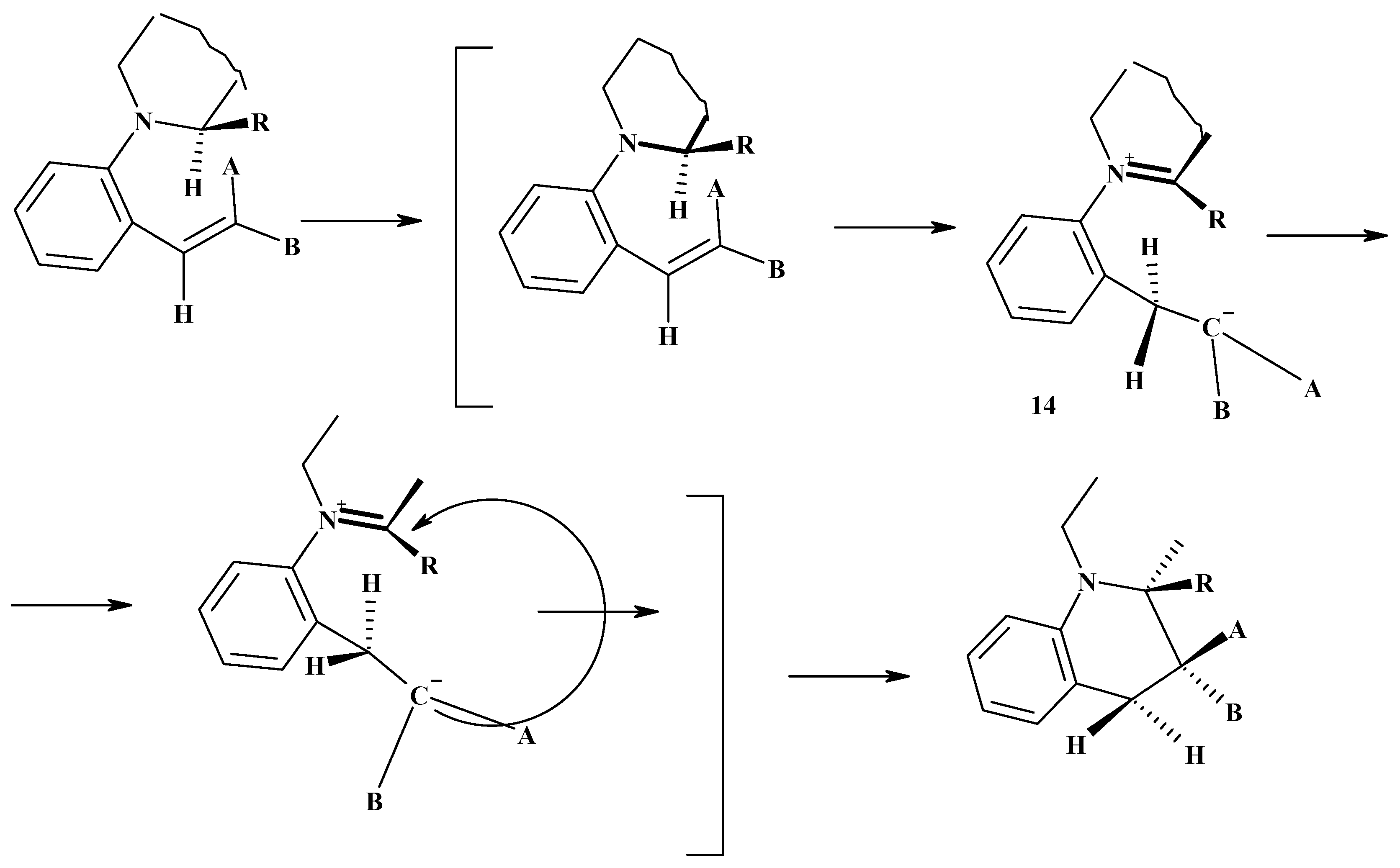
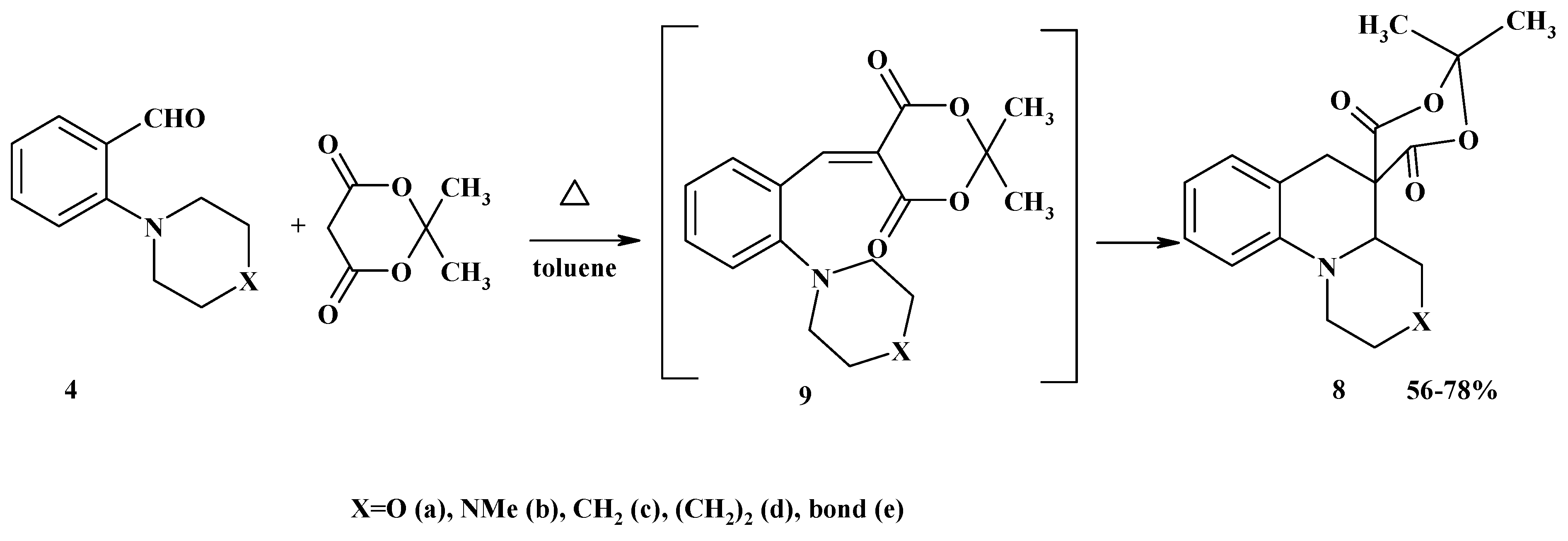
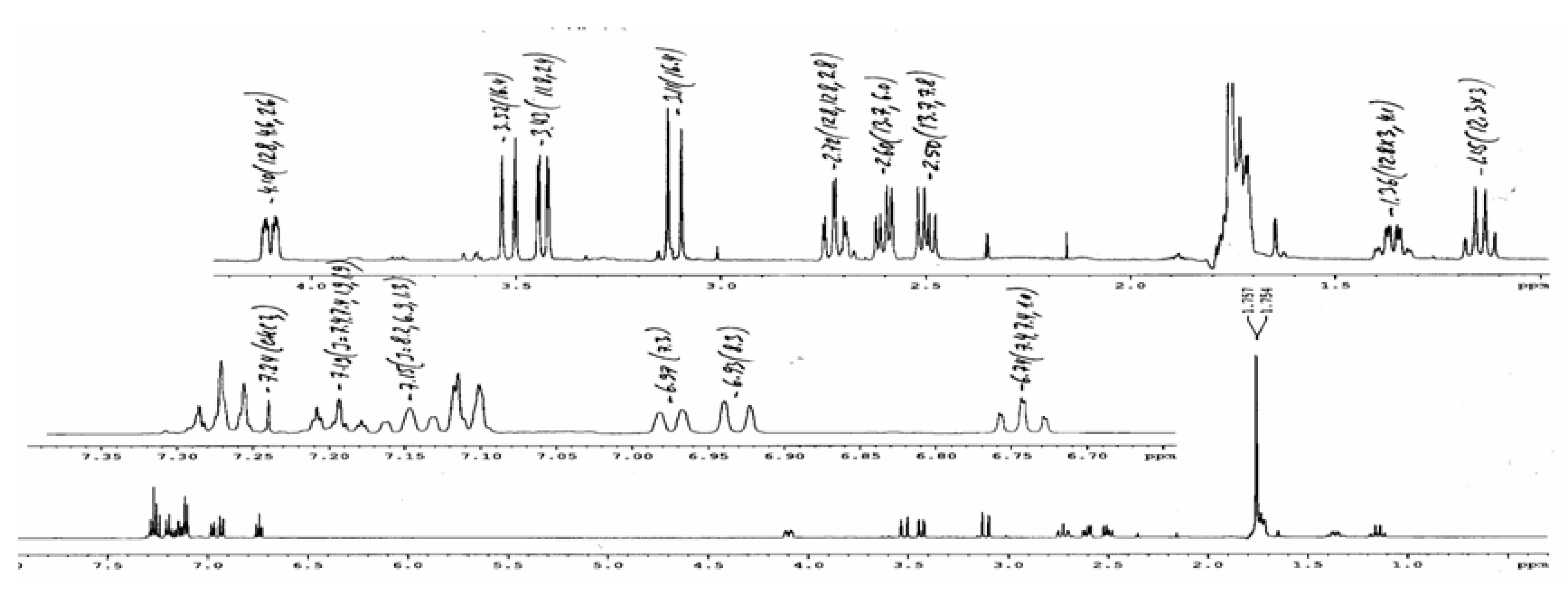
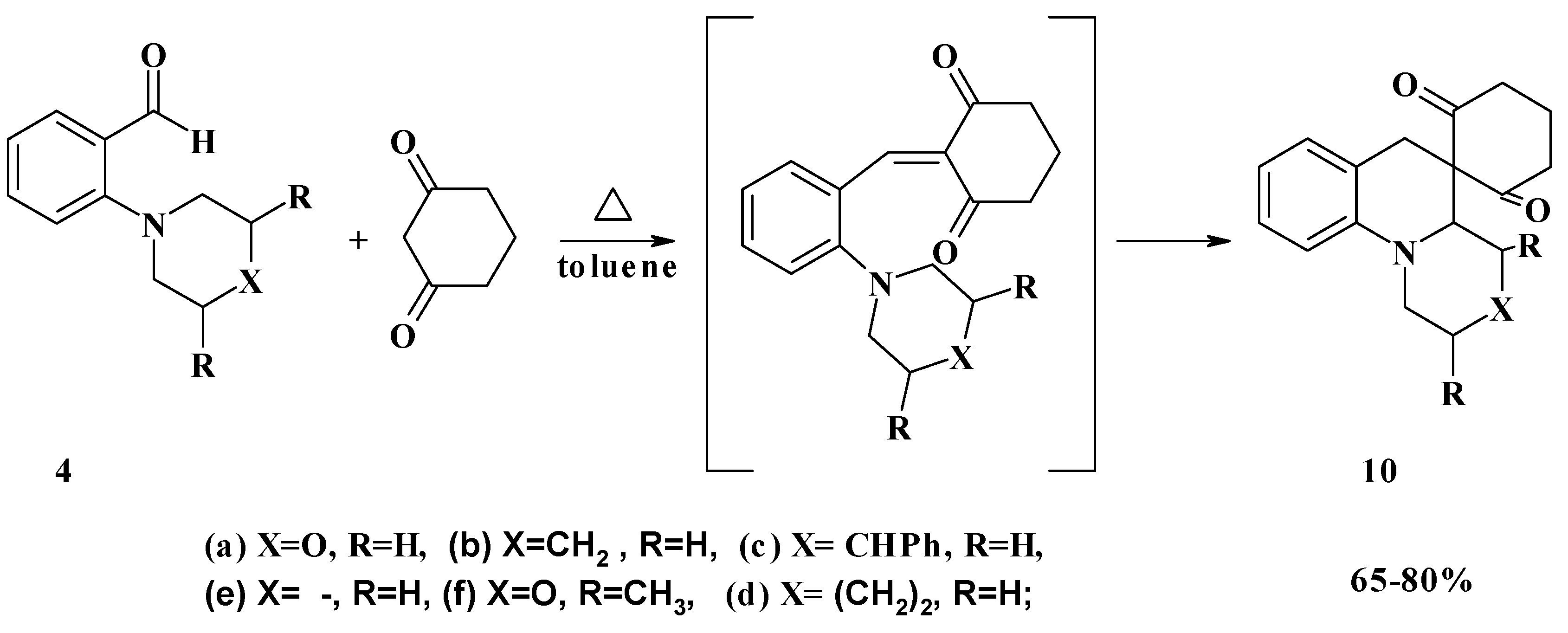
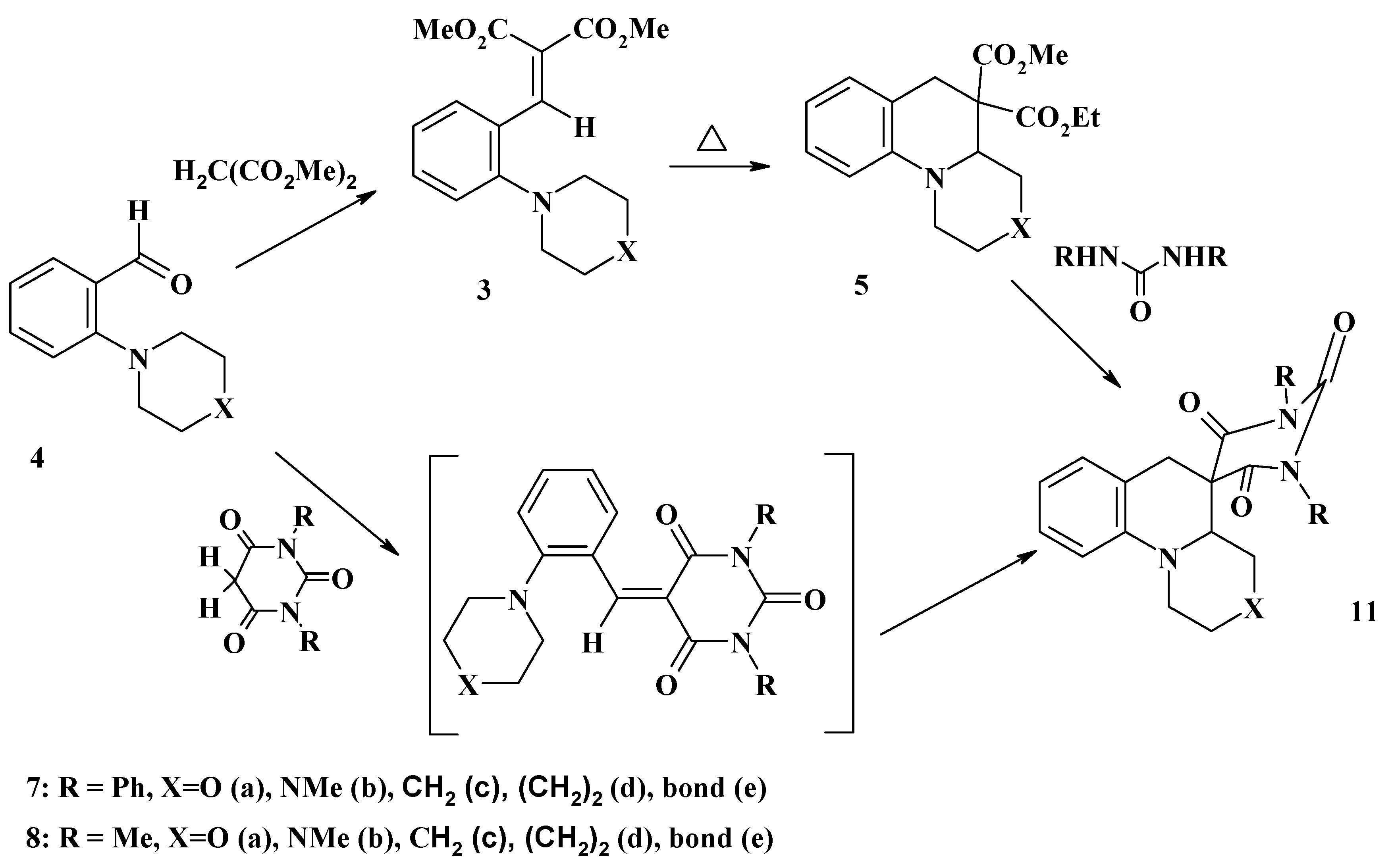
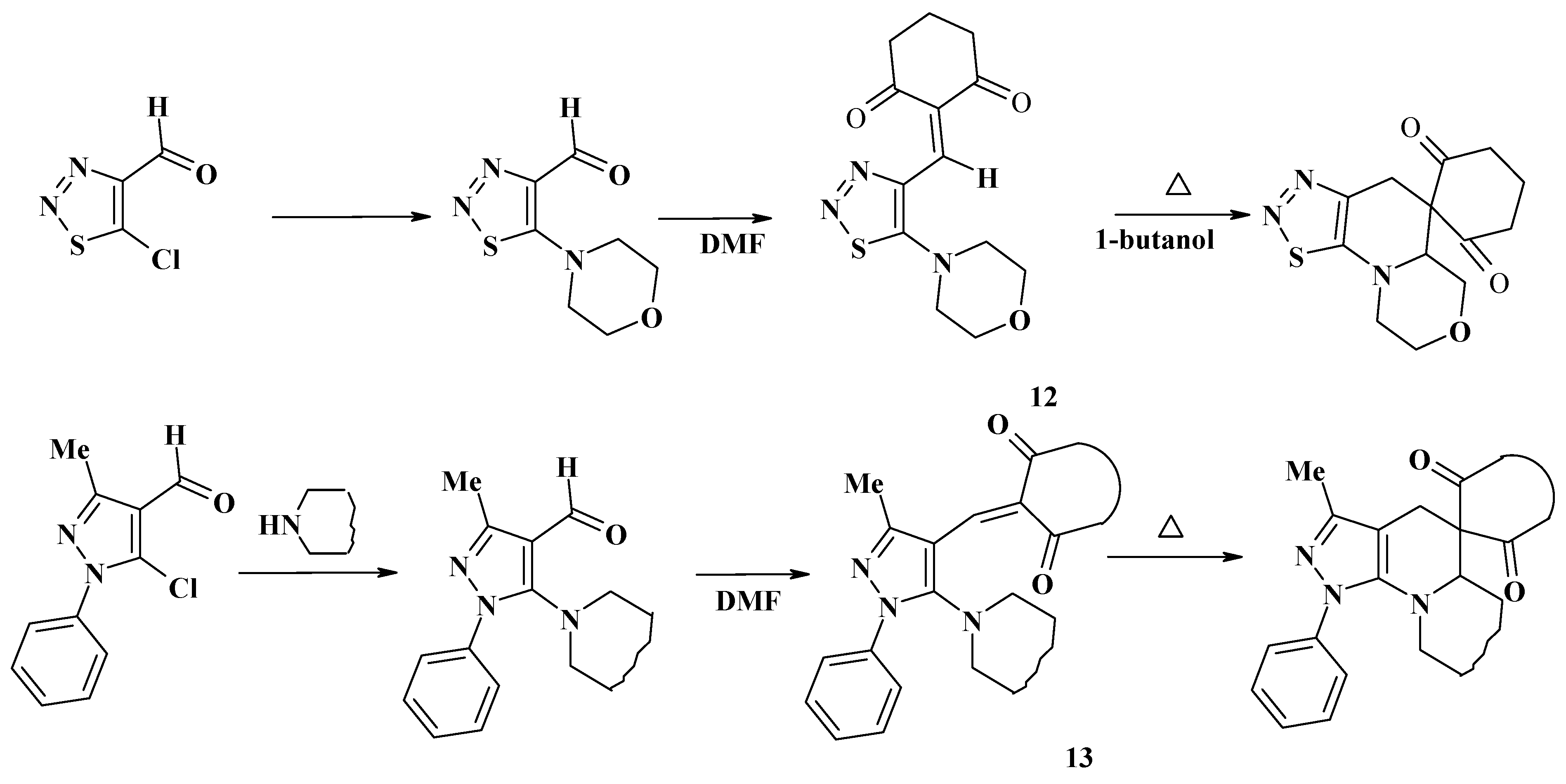
Conclusions
Acknowledgements
References
- Meth-Cohn, O.; Suschitzky, H. Adv. Heterocycl. Chem. 1972, 14, 211.
- Pinnow, J. Ber. Dtsch. Chem. Ges. 1895, 28, 3039.
- Seebach, D.; Enders, D. Angew. Chem. 1975, 87, 1.
- Fielden, R.; Meth-Cohn, O.; Suschizky, H. J. Chem. Soc., Perkin Trans. I 1973, 696.
- Gluhareva, T.V.; Morzherin, Yu.Yu.; Mokrushin, V.S. Chem. Heterocycl. Comp. (Engl. Transl.) 2000, 36, 107.
- Kirschke, K.; Möller, A.; Schmitz, E.; Kuban, R.J.; Schulz, B. Tetrahedon Lett. 1986, 27, 4281.
- Martin, J.; Meth-Cohn, O.; Suschitzky, H. Tetrahedron Lett. 1973, 4495.
- Tea Gokou, C.; Pradere, J.P.; Qiuniou, H. Synth. Commun. 1986, 16, 79.
- Akiba, M.; Kosugi, Y.; Takada, T. J. Org. Chem. 1978, 43, 4472.
- Suschitzky, H.; Walrond, R.E.; Hull, R. J. Chem.Soc., Perkin Trans. I 1977, 47.
- Falci, K.J.; Franck, R.W.; Smith, G.P. J. Org. Chem. 1977, 42, 3317. [PubMed]
- Fokin, E.P.; Russkikh, V.V. Zhur.Org. Khim. 1966, 2, 907.
- Nijuis, W.H.N.; Verboom, W.; Harkema, S.; Reinhoudt, R. Recl. Trav. Chim. Pays-Bas 1989, 108, 147.
- Verboom, W.; Reinhoudt, D.N. Rec. Trav. Chim. Pay-Bas 1990, 109, 311.
- Visser, G.W.; Verboom, W.; Benders, P.H.; Reinhoudt, D. J. Chem. Soc. Chem. Commun. 1982, 669.
- Verboom, W.; Lammerink, B.H.M.; Egberink, R.J.M.; Reinhoudt, D.N.; Harkema, S. J. Org. Chem. 1985, 50, 3797.
- D'yachenko, E.V.; Glukhareva, T.V.; Mezenova, E.V.; Zybina, N.A.; Lobodin, V.V.; Morzherin, Yu.Yu. Vestnik USTU-UPI. 2004, 7, 76–79.
- Lobodin, V.V.; Ovcharenko, V.V.; Pihlaja, K.; Morzherin, Yu.Yu.; Lebedev, A.T. Rapid Comm. Mass Spectrom. 2004, 18, 724–728.
- Ojea, V.; Muinelo, I.; Figueroa, M.C.; Ruiz, M.; Quintela, J.M. Synlett. 1995, 622.
- Kaval, N.; Dehaen, W.; Matyus, P.; Van der Eycken, E. Green Chem. 2004, 125.
- Melh-Cohn, O.; Suschiizky, H. Adv. Heterocycl. Chem. 1996, 65, 1.
- Verboom, W.; Reinhoudt, D.; Visser, R.; Harkema, S. J. Org.Chem. 1984, 49, 269.
- Dijksman, W.C.; Verboom, W.; Egberink, R.; Reinhoudt, D. J. Org. Chem. 1985, 50, 3791.
- Verboom, W.; Reinhoudt, D.N.; Visser, R.; Harkema, S. J. Org. Chem. 1989, 54, 209.
- Verboom, W.; Morzherin, Yu.; Kelderman, E.; Engbersen, J.F.J.; van Hummel, G.J.; Harkema, S.; Reinhoudt, D.N. Recl. Trav. Chim. Pays-Bas 1993, 112, 549.
- Glukhareva, T.V.; D'yachenko, E.V.; Morzherin, Yu.Yu. Prog. Org. Synth. (Ekaterinburg). 2003, 61.
- Nijuis, W.; Verboom, W.; Reinhoudt, D.; Harkema, S. J. Am. Chem. Soc. 1987, 109, 3136.
- Nijhuis, W.; Verboom, W.; Abu El-Fadl, A.; Harkema, S.; Reinhoudt, D. J. Org. Chem. 1989, 54, 209.
- Glukhareva, T.V.; D'yachenko, E.V.; Morzherin, Yu.Yu. Chem. Heterocycl. Comp. (Engl. Transl.) 2002, 38, 1426.
- D´yachenko, E.V.; Glukhareva, T.V.; Nikolaenko, E.F.; Tkachev, A.V.; Morzherin, Yu.Yu. Russ. Chem. Bull. (Intern. Ed.) 2004, 1194.
- D'yachenko, E.V.; Glukhareva, T.V.; Morzherin, Yu.Yu. Chem. Heterocycl. Comp. (Engl. Transl.) 2003, 39, 1532.
- Morzherin, Y.Y. Dissertation Tesis, Ekaterinburg (Russia), 2004.
- Samples Availability: Available from the authors.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
D’yachenko, E.; Glukhareva, T.; Dyudya, L.; Eltsov, O.; Morzherin, Y. The tert-Amino Effect in Heterocyclic Chemistry. Synthesis of Spiro Heterocycles. Molecules 2005, 10, 1101-1108. https://doi.org/10.3390/10091101
D’yachenko E, Glukhareva T, Dyudya L, Eltsov O, Morzherin Y. The tert-Amino Effect in Heterocyclic Chemistry. Synthesis of Spiro Heterocycles. Molecules. 2005; 10(9):1101-1108. https://doi.org/10.3390/10091101
Chicago/Turabian StyleD’yachenko, E., T. Glukhareva, L. Dyudya, O. Eltsov, and Y. Morzherin. 2005. "The tert-Amino Effect in Heterocyclic Chemistry. Synthesis of Spiro Heterocycles" Molecules 10, no. 9: 1101-1108. https://doi.org/10.3390/10091101



