Synthesis of Some New bis-Schiff Bases of Isatin and 5-Fluoroisatin in a Water Suspension Medium
Abstract
:Introduction
Results and Discussion
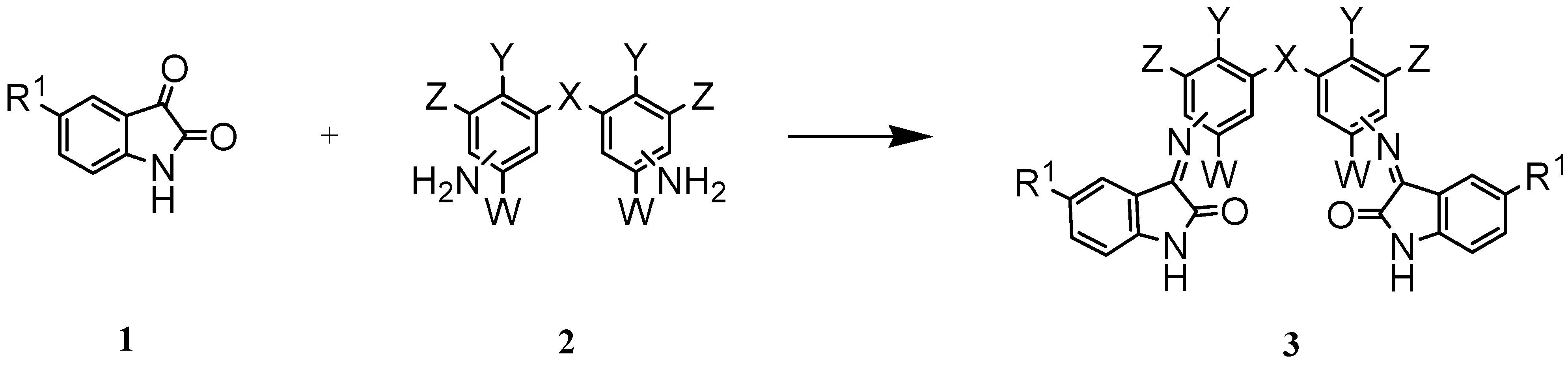
| 3 | R1 | X | Y | W | Z | Position of C=N relative to X | Reaction time/h | Yield % |
| a* | H | CH2 | H | H | H | 4,4′ | 22 | 98.0 |
| b | H | CH2 | H | H | H | 3,3′ | 30 | 72.7 |
| c | H | O | H | H | H | 3,4′ | 45 | 80.0 |
| d* | H | O | H | H | H | 4,4′ | 24 | 92.9 |
| e | F | CH2 | H | H | H | 4,4′ | 17 | 82.1 |
| f | F | CH2 | H | H | H | 3,3′ | 22 | 99.0 |
| g | F | O | H | H | H | 4,4′ | 17 | 90.2 |
| h | H | CH2 | Cl | Et | Et | 4,4′ | 48 | 70.0 |
Experimental
General:
Typical procedure for the preparation of bis-Schiff bases of isatin and 5-fluoroisatin
Acknowledgments
References
- Macho, V.; Kralik, M.; Hudec, J.; Cingelova, J. J. Mol. Catal. A: Chem. 2004, 209, 69. [CrossRef]
- Bey, P.; Vevert, J. P. Tetrahedron Lett. 1977, 18, 1455. [CrossRef]
- Lucas, R. A.; Dickel, D. F.; Dziemian, M. J.; Hensle, B. L.; Mcphillarney, H. B. J. Am. Chem. Soc. 1960, 82, 5688.
- Fleet, G. W. J.; Fleming, I. J. Chem. Soc. 1969, 1758.
- Bezas, B.; Zervas, L. J. Am. Chem. Soc. 1961, 83, 719.
- Adams, J. P. J. Chem. Soc., Perkin Trans. 1 2000, 125. [CrossRef]
- Layer, R. W. Chem. Rev. 1963, 63, 489.Abbaspour, A.; Esmaeilbeig, A. R.; Jarrahpour, A. A.; Khajeh, B.; Kia, R. Talanta 2002, 58, 397.
- Jarrahpour, A. A.; Motamedifar, M.; Pakshir, K.; Hadi, N.; Zarei, M. Molecules 2009, 9, 815.Alexander, V. Chem. Rev. 1995, 95, 273.
- Higuchi, M.; Yamamoto, K. Org. Lett. 1999, 1, 1881.
- Sridhar, S. K.; Saravanan, M.; Ramesh, A. Eur. J. Med. Chem. 2001, 36, 615.
- Deshmukh, A. R. A. S.; Jayanthi, A.; Puranik, V.G.; Bhawal, B. M. Synthesis 2004, 1249.Karupaiyan, K.; Puranik, V. G.; Deshmukh, A. R. A. S.; Bhawal, B. M. Tetrahedron 2000, 56, 8555.
- Kunz, H.; Pfrengle, W.; Ruck, K.; Sager, W. Synthesis 1991, 1039.Kunz, H.; Sager, W. Angew. Chem. Int. Ed. Engl. 1987, 26, 557.
- Vazzana, I.; Terranova, E.; Mattioli, F.; Sparatore, F. Arkivoc 2004, 364.
- Weingarten, H.; Chupp, J. P.; White, W. A. J. Org. Chem. 1967, 32, 3246.
- Bonett, R.; Emerson, T. R. J. Chem. Soc. 1965, 4508. [CrossRef]Roelofsen, D. P.; van Bekkum, H. Recl. Trav. Chim. Pays-Bays 1972, 91, 605.
- Tanaka, T.; Toda, F. Chem. Rev. 2000, 100, 1025.Varma, R. S. Green Chem. 1999, 1, 43.
- Varma, R. S.; Dahiya, R.; Kumar, S. Tetrahedron Lett. 1997, 38, 2039. [CrossRef]
- Schmeyers, J.; Toda, F.; Boy, J.; Kaupp, J. J. Chem. Soc., Perkin Trans.1 1998, 989.
- Bergman, Y.; Perlmutter, P.; Thienthong, N. Green Chem. 2004, 6, 539.
- Bader, B. D.; Vilceanu, R. Rev. Roum. Chim. 1972, 17, 1991.Vilceanu, R.; Bader, B. D.; Radulescu, M.; Marinescu, M. Rev. Roum. Chim. 1973, 18, 1225.
- Sample availability: Contact the authors or MDPI
© 2006 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes
Share and Cite
Jarrahpour, A.A.; Khalili, D. Synthesis of Some New bis-Schiff Bases of Isatin and 5-Fluoroisatin in a Water Suspension Medium. Molecules 2006, 11, 59-63. https://doi.org/10.3390/11010059
Jarrahpour AA, Khalili D. Synthesis of Some New bis-Schiff Bases of Isatin and 5-Fluoroisatin in a Water Suspension Medium. Molecules. 2006; 11(1):59-63. https://doi.org/10.3390/11010059
Chicago/Turabian StyleJarrahpour, A. A., and D. Khalili. 2006. "Synthesis of Some New bis-Schiff Bases of Isatin and 5-Fluoroisatin in a Water Suspension Medium" Molecules 11, no. 1: 59-63. https://doi.org/10.3390/11010059
APA StyleJarrahpour, A. A., & Khalili, D. (2006). Synthesis of Some New bis-Schiff Bases of Isatin and 5-Fluoroisatin in a Water Suspension Medium. Molecules, 11(1), 59-63. https://doi.org/10.3390/11010059




