Synthesis and Characterization of Dimethacrylate Monomer with High Molecular Weight for Root Canal Filling Materials
Abstract
:Introduction
Results and Discussion
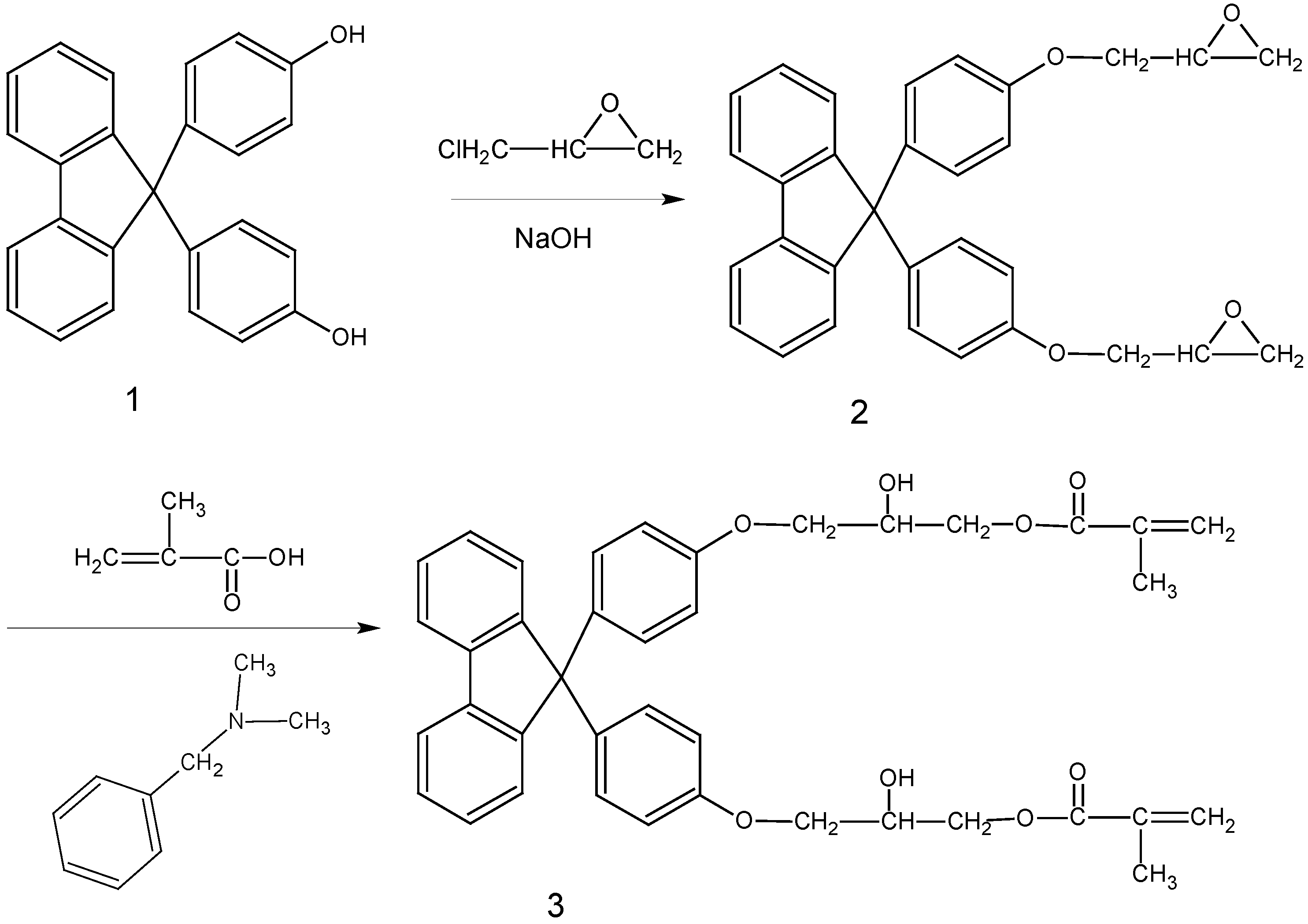
 , 2924 cm-1 (O-CH2) and 3002 cm-1 (epoxide CH and CH2) while the absorption peak at 3480 cm-1 (-OH) of starting compound 1 disappeared. This indicated that the hydroxyl groups in compound 1 had reacted completely with epichlorohydrin and glycidyl ether groups had been introduced into compound 1. The 1H-NMR spectrum of compound 2 could be unambiguously assigned considering the features of 1H-NMR spectra and the signal intensities. In the mass spectrum of compound 2, a peak with m/z = 462 also supported the proposed structure.
, 2924 cm-1 (O-CH2) and 3002 cm-1 (epoxide CH and CH2) while the absorption peak at 3480 cm-1 (-OH) of starting compound 1 disappeared. This indicated that the hydroxyl groups in compound 1 had reacted completely with epichlorohydrin and glycidyl ether groups had been introduced into compound 1. The 1H-NMR spectrum of compound 2 could be unambiguously assigned considering the features of 1H-NMR spectra and the signal intensities. In the mass spectrum of compound 2, a peak with m/z = 462 also supported the proposed structure.Conclusions
Experimental
General
| Compound | 1H-NMR δ ppm | FT-IR (KBr) cm-1 | MS m/z (%) | Elemental analysis Formula Calculated % Found % |
| 1 | − | 3480 (OH) | 350 (100), 333 (9.43), 257 (39.17) | − |
| 2 | 6.775-7.776 (16H, Ar-O-) 4.134-4.170 (dd, J = 11 Hz, 3.3 Hz, 2H, ) 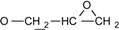 3.897-3.939 (dd, J= 11 Hz, 5.5Hz, 2H, ) 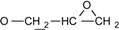 3.301-3.338 (m, 2H, 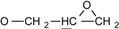 ) )2.728-2.734 (dd, J = 4.8 Hz, 2.5 Hz, 2H, 4H, 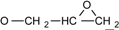 ) )2.873-2.895 (t, J = 4.5 Hz, 2H, 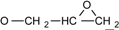 ) ) | 3002 ( 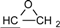 and and 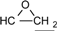 ) )2924 (O-CH2) 910 ( 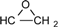 ) ) | 462 (100), 405 (17.82), 348 (3.59), 257 (16.73) | C31H26O4 C: 80.52 H: 5.63 C: 79.99 H: 5.66 |
| 3 | 6.708-7.744 (16H,Ar-O-) 6.112 (2H,-C=C-H) 5.556-5.578(2H,-C=C-H’) 3.925-4.324 (12H,-O-CH2-CHOH-CH2) 1.905-1.928 (6H,-C-CH3) | 3413 (OH) 2952 (CH3) 1717 (C=O) 1635 (C=C) | 634 (7.81), 566 (2.76), 548 (14.18), 492 (5.13), 423 (4.08), 349 (8.25), 257 (31.73), 143 (100), 69 (31.84) | C39H38O8 C: 73.82 H: 5.99 C: 74.04 H: 6.01 |
Preparation of 9,9′-bis(4-oxiranylmethoxyphenyl)fluorene (2)
Preparation of 9,9′-bis[4-(2′-hydroxy-3′-methacryloyloxypropoxy)phenyl] fluorene (3)
References
- He, Y. L. Plastic material for dental surgery and its applied formulations. Plast. Sci. Technol. 1999, 23–26. [Google Scholar]
- Bowen, R.L. Dental filling material. US Pat. 3066112 1962. [Google Scholar]
- Suprabha, B.S; Sudha, P.; Vidya, M. A comparative evaluation of sealing ability of root canal sealers. Indian J. Dent. Res. 2002, 13, 31–36. [Google Scholar]
- Shipper, G.; Orstavik, D.; Teixeira, F.B.; Trope, M. An evaluation of microbial leakage in roots filled with a thermoplastic synthetic polymer-based root canal filling material (ResilonTM). J. Endod. 2004, 30, 342–347. [Google Scholar] [CrossRef]
- Heintze, S.D.; Cavalleri, A.; Forjanic, M.; Zellweger, G.; Rousson, V. A comparison of three different methods for the quantification of the in vitro wear of dental materials. Dent. Mater. 2006, 22, 1051–1062. [Google Scholar] [CrossRef]
- Versiani, M. A.; Carvalho-junior, J. R.; Padilha, M.; Lacey, S.; Pascon, E. A.; Sousa-Neto, M. D. A comparative study of physicochemical properties of AH PlusTM and EpiphanyTM root canal sealants. Int. Endod. J. 2006, 39, 464–471. [Google Scholar] [CrossRef]
- Venhoven, B.A.M.; De Gee, A.J.; Davidson, C.L. Polymerization contraction and conversion of light-curing BisGMA based methacrylate resins. Biomaterials 1993, 14, 871–875. [Google Scholar] [CrossRef]
- Khatri, C. A.; Stansbury, J. W.; Schultheisz, C. R.; Antonucci, J. M. Synthesis, characterization and evaluation of urethane derivatives of Bis-GMA. Dent. Mater. 2003, 19, 584–588. [Google Scholar]
- Ge, J.; Trujillo, M.; Stansbury, J. Synthesis and photopolymerization of low shrinkage methacrylate monomers containing bulky substituent groups. Dent. Mater. 2005, 21, 1163–1169. [Google Scholar] [CrossRef]
- Ahn, K. D.; Chung, C. M.; Kim, Y. H. Synthesis and photopolymerization of multifunctional methacrylates derived from Bis-GMA for dental applications. J. Appl. Polym. Sci. 1999, 71, 2033–2037. [Google Scholar] [CrossRef]
- Chung, C. M.; Kim, J. G.; Kim, M. S.; Kim, K. M; Kim, K. N. Development of a new photocurable composite resin with reduced curing shrinkage. Dent. Mater. 2002, 18, 174–178. [Google Scholar]
- Chung, C. M.; Kim, M. S.; Kim, J. G.; Jang, D. O. Synthesis and photopolymerization of trifunctional methacrylates and their application as dental monomers. J. Biomed. Mater. Res. 2002, 62, 622–627. [Google Scholar] [CrossRef]
- Culbertson, B. M.; Tiba, A.; Sang, J.; Liu, Y. N. Synthesis, characterization and evaluation of new fluorene-based dimethacrylates for formulating dental composites. Polym. Adv. Technol. 1999, 10, 275–281. [Google Scholar] [CrossRef]
- Okada, S. Curable acrylic resin compositions with good light resistance. Jpn. Kokai Tokkyo Koho JP 2004315744 2004. [Google Scholar]
- Ishii, K.; Yokoshima, M. Resin compositions for optical materials, optical disk materials and cured products thereof. Jpn. Kokai Tokkyo Koho JP 04337307 1992. [Google Scholar]
- Lilja, J.; Murzin, D.Yu.; Salmi, T.; Aumo, J.; Mäki-Arvela, P.; Sundell, M. Esterification of different acids over heterogeneous and homogeneous catalysts and correlation with the Taft equation. J. Mol. Catal. A: Chem. 2002, 182–183, 555–563. [Google Scholar]
- Sample Availability: Available from the authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Liu, F.; He, J.-w.; Lin, Z.-m.; Ling, J.-q.; Jia, D.-m. Synthesis and Characterization of Dimethacrylate Monomer with High Molecular Weight for Root Canal Filling Materials. Molecules 2006, 11, 953-958. https://doi.org/10.3390/11120953
Liu F, He J-w, Lin Z-m, Ling J-q, Jia D-m. Synthesis and Characterization of Dimethacrylate Monomer with High Molecular Weight for Root Canal Filling Materials. Molecules. 2006; 11(12):953-958. https://doi.org/10.3390/11120953
Chicago/Turabian StyleLiu, Fang, Jing-wie He, Zheng-mei Lin, Jun-qi Ling, and De-min Jia. 2006. "Synthesis and Characterization of Dimethacrylate Monomer with High Molecular Weight for Root Canal Filling Materials" Molecules 11, no. 12: 953-958. https://doi.org/10.3390/11120953
APA StyleLiu, F., He, J.-w., Lin, Z.-m., Ling, J.-q., & Jia, D.-m. (2006). Synthesis and Characterization of Dimethacrylate Monomer with High Molecular Weight for Root Canal Filling Materials. Molecules, 11(12), 953-958. https://doi.org/10.3390/11120953




