Introduction
1,2,4-Triazines and their condensed derivatives form an important class of heteroaromatic compounds with various biochemically interesting properties and pharmacologically significant activities [
1,
2]. We have recently explored the mechanism of the cyclization of the monocyclic compound 3-amino-6-hydrazino-1,2,4-triazin-5(2
H)-one (
1) to form the heterobicyclic 6-amino-3-substituted-1,2,4-triazolo[3,4-
f][1,2,4]triazin-8(7
H)-ones
2 [
3]. This convenient reaction of
1 to afford
2 (
Scheme 1) had been previously described by Lovelette [
4]. As a part of our ongoing program, the compound 3-amino-2-benzyl-6-hydrazino-1,2,4-triazin-5(2
H)-one (
3) was treated with a variety of one-carbon fragment reagents (formic acid, glacial acetic acid or cyanogen bromide) and no cyclization was observed [
5]. In this paper we report an unexpected ring closed product
4 obtained when compound
3 is treated with carbon disulfide. As we know, many aza/deaza analogues of purine have attracted considerable interest due to their biological activities. Compound
4 contains a 6-amino-1,2,4-triazolo- [3,4-
f][1,2,4]triazin-8(7
H)-one moiety (4,8-diaza-9-deazaguanine), which is an isosteric isomer of guanine. In the last decade numerous fused 1,2,4-triazines have been synthesized and screened
in vitro, revealing their anti-HIV and anti-cancer activities [
6,
7,
8,
9,
10,
11,
12,
13]. Meanwhile, fused 1,2,4-triazoles have acquired considerable importance because of their CNS depressant, anti-allergy, anti-inflammatory and antimicrobial properties [
14,
15,
16,
17]. However, the 3-substituted derivatives of 6-amino-1,2,4-triazolo- [3,4-
f][1,2,4]triazin-8(7
H)-one have seldom been reported.
Results and Discussion
When compound
3 was refluxed with carbon disulfide in a 1:1 water/pyridine mixture an unexpected ring closed product
4 was obtained (
Scheme 2). An intense IR absorption at 1735 cm
-1 suggested the carbonyl absorption of fused triazines and confirmed the occurrence of ring closure [
3,
4,
18]. The
1H-NMR spectrum showed two singlets at δ
H 4.50 and 6.50 ppm, corresponding to the benzyl group methylene protons and the 6-amino group, respectively. The remaining multiplets at δ
H 7.30 to 7.37 ppm were attributed to the aromatic protons of the benzyl group, while the broad singlet at δ
H 11.61 ppm corresponded to the NH proton of the 1,2,4-triazinone ring amide. The location of the 3-benzylmercapto group of
4 at C-3 was established by 2D-NMR. The long-range
1H-
13C heteronuclear correlation (HETCOR) NMR revealed the connectivity of the methylene protons (δ
H 4.50 ppm) on the benzyl group to C-3 (δ
C 144.86 ppm) in the 1,2,4-triazolo ring. Additional supporting evidence for
4 was found in the high-resolution mass spectrum showing the molecular ion at m/z 274.0635, confirming the precise molecular formula as C
11H
10N
6OS (calcd. 274.0637).
Further confirmation of the structure of product
4 was obtained from its synthesis
via an alternative approach, by reacting 6-amino-3(2
H)mercapto-1,2,4-triazolo[3,4-
f][1,2,4]triazin-8(7
H)-one (
7) with benzyl bromide in methanolic ammonia (
Scheme 2). The resulting nucleophilic substitution product was a material identical in every respect with compound
4. Finally, the structure of
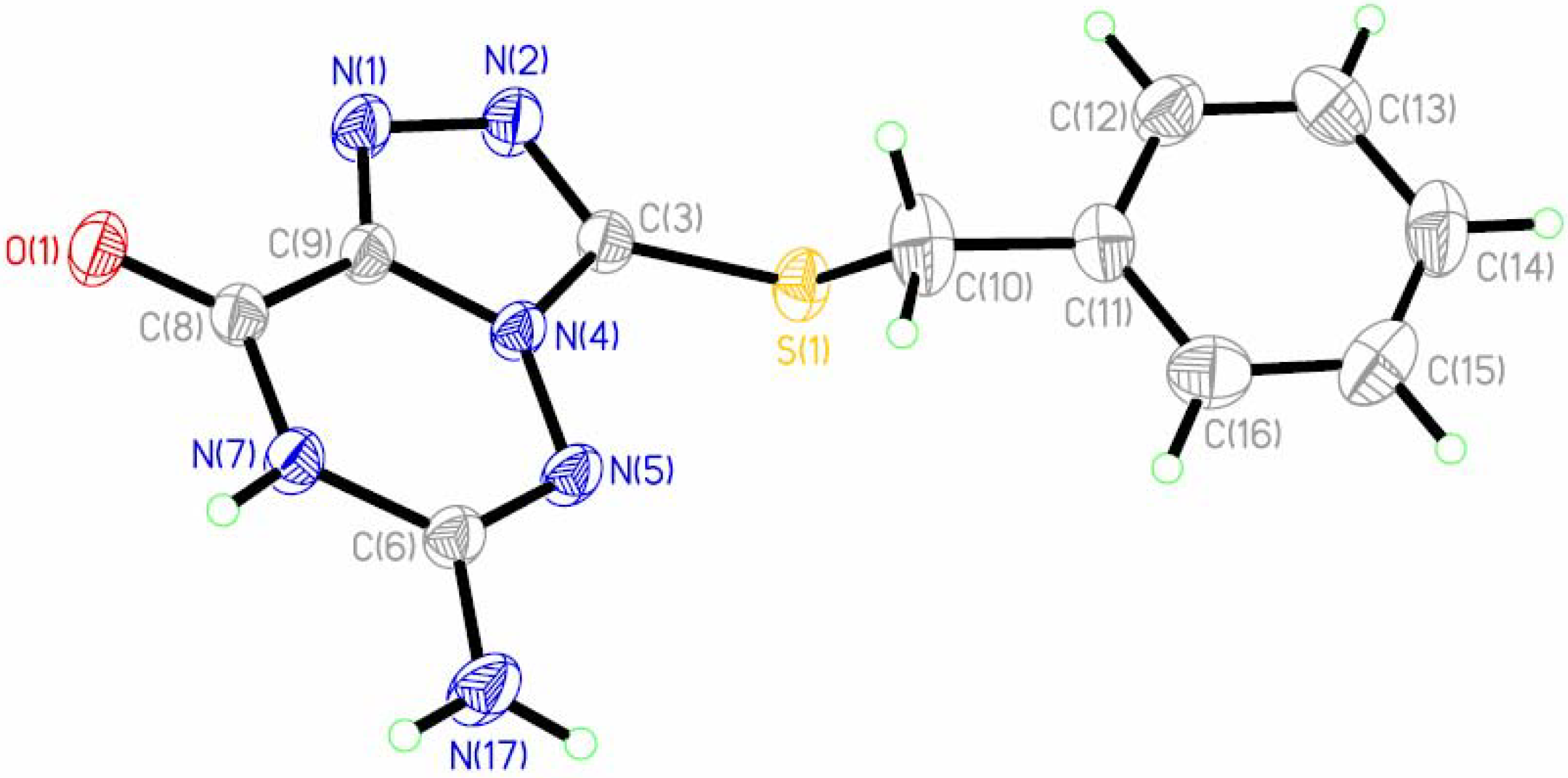
4 was unambiguously confirmed by X-ray crystallography, revealing the structural framework as a 1,2,4-triazole five-membered ring fused at C(9)-N(4) with a 1,2,4-triazine six-membered ring (
Figure 1). The most contributing prototropic tautomerism of
4 is the amino-oxo form 7
H-tautomer (not the 5
H-tautomer), similar to the tautomerism of 6-amino-3-ethyl-1,2,4-triazolo[3,4-
f][1,2,4]-triazin- 8(7
H)-one, as previously reported by us [
19]. The benzylmercapto group was substituted at C-3 showing an exoextensibility. Taken together,
4 was assigned as 6-amino-3-benzylmercapto-1,2,4- triazolo[3,4-
f][1,2,4]-triazin-8(7
H)-one. The ring closed compound
5 and the hydrazinecarbodithioic acid compound
6 were not observed (
Scheme 2).
Figure 1.
ORTEP drawing and atom labelling scheme of the compound 4 with thermal ellipsoids drawn at the 50% probability level.
Figure 1.
ORTEP drawing and atom labelling scheme of the compound 4 with thermal ellipsoids drawn at the 50% probability level.
The ORTEP drawing for the title compound is depicted in
Figure 1. The molecular packing is shown in
Figure 2. In the structure of
4 the short bonds 1.310(2) Å (N(5)-C(6)), 1.320(2) Å (N(1)-C(9)) and 1.325(2) Å (N(2)-C(3)) have an appreciable double-bond character. The bond lengths 1.732(2) Å of C(3)-S(1) and 1.214(2) Å of C(8)-O(1) are shorter than the bond lengths of C
ar-S(2) (1.773 Å) and C
sp2=O(1) (1.240 Å) in
δ-lactams [
20], respectively. These may be attributed to the electron abstraction by the
π-deficient heterobicyclic ring. The bond length 1.340(2) Å between C(6)-N(17) is shorter than 1.355 Å of C
ar-NH
2 (N
sp2: planar) [
20]. Meanwhile, the 119.15(15) value of the N(5)-C(6)-N(17) angle, close to 120°, confirms the
sp2 hybridization of the nitrogen atom, which suggests that the 6-amino group strongly donates the unpaired electrons and resonates with the [1,2,4]triazolo[3,4-
f][1,2,4]triazinone ring. The mean plane of 1,2,4-triazolo[3,4-
f][1,2,4]triazine ring forms a dihedral angle of 20.27° with the benzyl group benzene ring.
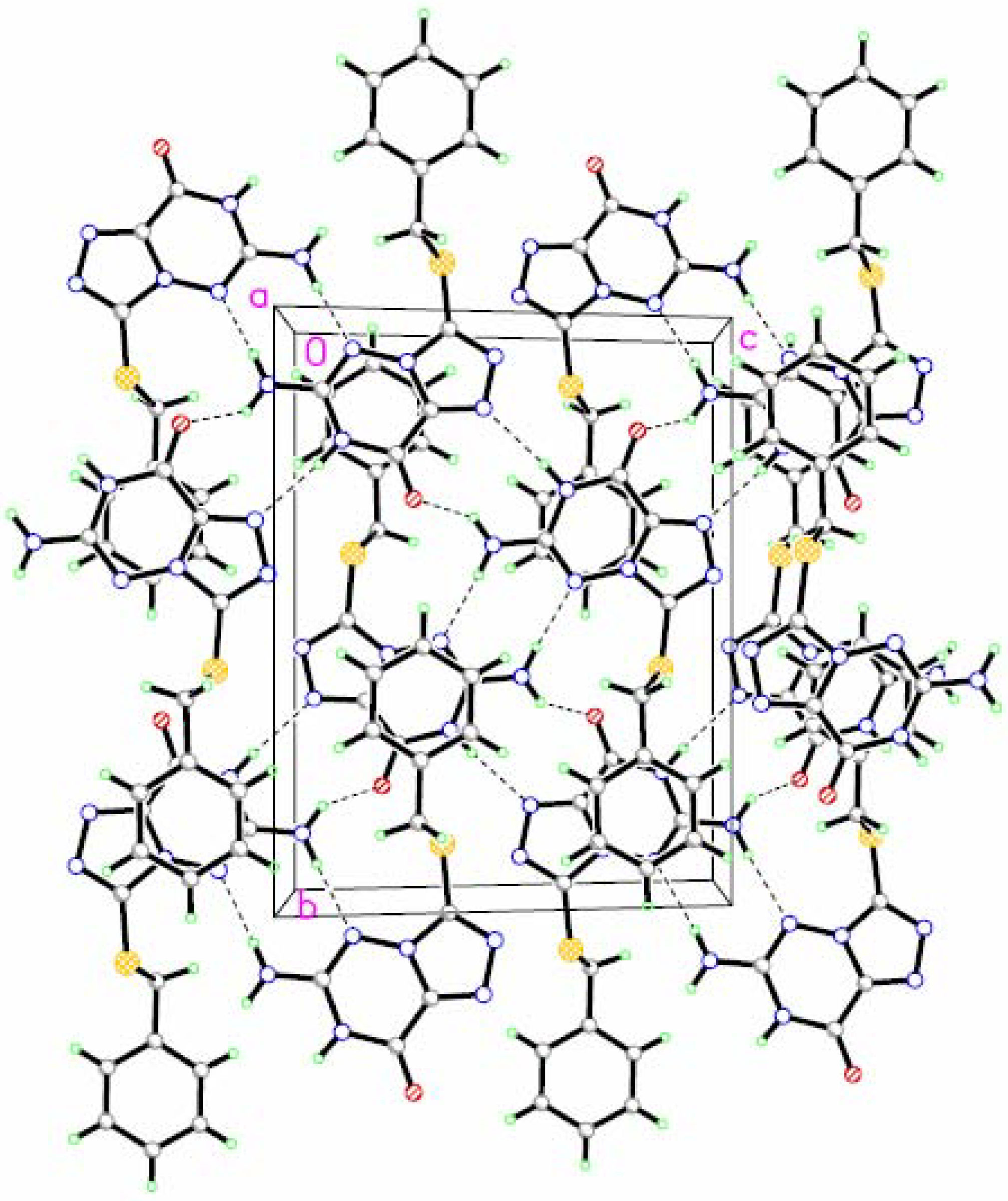
Figure 2.
A perspective drawing of the packing arrangement of compound 4. Dashed lines are intermolecular N-H···N and N-H···O hydrogen bonds.
Figure 2.
A perspective drawing of the packing arrangement of compound 4. Dashed lines are intermolecular N-H···N and N-H···O hydrogen bonds.
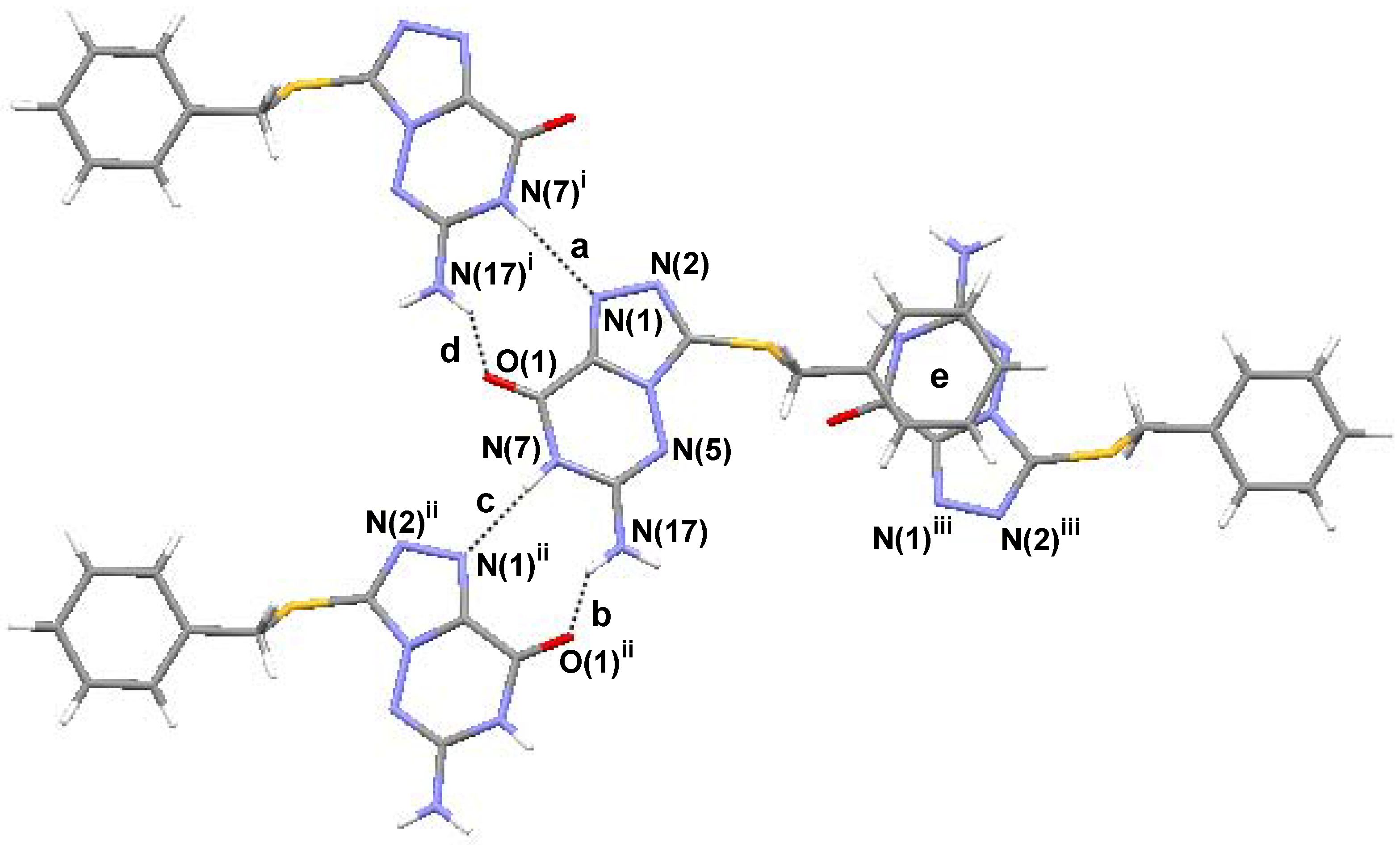
Analysis of the molecular packing in the unit cell reveals that each molecule is linked with two other molecules by intermolecular hydrogen bonds (
Figure 3 and
Table 1). Each title molecule is linked into two
(9) graph set associations (
Figure 3, notation [a], [d] and [b], [c]), each
via one N-H···N hydrogen bond and one N-H···O hydrogen bond. Assignment of the H-bond descriptors is based on the graph-set theory [
21]. The structure is further stabilized by π-π stacking (
Figure 3 notation [e]) interactions, which results in the centroid···centroid distance (3.695 Å) being that between the benzene ring of the molecule at
x,
y,
z and the 1,2,4-triazine ring of the molecule at 1
-x, 0.5+
y, 1.5–
z (iii) ([e]). Molecular graphics and hydrogen bond geometry were obtained using the program Mercury (version 1.4, CCDC, Cambridge, UK).
Figure 3.
A part of the crystal structure of the title compound, showing the molecule stacking (notation [e]) direction along the
a-view. Broken lines indicate the intermolecular hydrogen bonding patterns. For notation and symmetry codes see
Table 1.
Figure 3.
A part of the crystal structure of the title compound, showing the molecule stacking (notation [e]) direction along the
a-view. Broken lines indicate the intermolecular hydrogen bonding patterns. For notation and symmetry codes see
Table 1.
Table 1.
Hydrogen bond geometry in compound 4
Table 1.
Hydrogen bond geometry in compound 4
| Notation | D-H···A | D-H (Å) | H···A (Å) | D···A (Å) | Length-VdW | D-H···A(°) |
|---|
| a | N(7)-H(7A)i···N(1) | 0.860 | 2.104 | 2.961 | –0.646 | 173.94 |
| b | N(17)-H(17B)···O(1)ii | 0.860 | 2.311 | 2.976 | –0.409 | 134.34 |
| c | N(7)-H(7A)···N(1)ii | 0.860 | 2.104 | 2.961 | –0.646 | 173.94 |
| d | N(17)-H(17B)i···O(1) | 0.860 | 2.311 | 2.976 | –0.409 | 134.34 |
Experimental
General
Melting points were determined on a YANACO micromelting point apparatus and are uncorrected.
1H- and
13C-NMR spectra (in DMSO-
d6, internal standard TMS) were taken on a Varian Unity 400 (400 MHz) spectrophotometer. For the assignments of signals, standard and long-range
1H-
13C heteronuclear chemical shift correlation (HETCOR) 2D NMR experiments were used. Chemical shifts are given in the δ-scale (ppm). Infrared spectra (IR) were recorded on a Perkin-Elmer FTIR 1650 spectrophotometer with KBr discs. MS and HRMS spectra were obtained on a Quattro VG-5022 spectrometer and VG 70-250S GC/MS, respectively, with an ionization potential of 70 eV. Elemental analyses (EA) were performed on a Heraeus CHN-O-Rapid elemental analyzer. 3-Amino-2-benzyl- 6-hydrazino-1,2,4-triazin-5(2
H)-one (3) and 6-amino-3-thio(2
H)-1,2,4-triazolo- [3,4-
f][1,2,4]triazin- 8(7
H)-one (7) were prepared according to the methods described by Hwang
et al. [
3] and Lovelette [
4], respectively.
Syntheses of 6-Amino-3-benzylmercapto-1,2,4-triazolo[3,4-f][1,2,4]triazin-8(7H)-one (4)
Method A: Compound 3 (0.35 g, 1.5 mmol) was suspended in a 1:1 pyridine/water mixture (15 mL), then carbon disulfide (0.16 g, 2.1 mmol) was added and the mixture was refluxed for 12 hours. Evaporation of the solvent under vacuum afforded a crude solid which was recrystallized from water to give 4 (0.16 g, 44%), m.p. 229-230 °C; IR (νmax, cm–1): 3431, 3330, 3016, 1735 (C=O), 1630, 1404, 1099; 1H-NMR: δ 4.50 (s, 2H, S-CH2-C6H5), 6.50 (s, 2H, NH2), 7.30-7.37 (m, 5H, C6H5), 11.61 (br s, 1H, NH); 13C-NMR: δ 34.79 (S-CH2-C6H5), 127.39, 128.42, 128.89, 137.24 (C6H5), 141.03 (C-8a), 144.86 (C-3), 150.67 (C-6), 151.59 (C-8); MS [EI]: m/z 274 (M+, 1), 214 (19), 197 (3), 165 (1), 106 (40), 91 (100), 77 (7), 70 (6), 65 (31); HRMS: m/z Calcd for C11H10N6OS: 274.0637. Found: 274.0635; Anal. Calcd for C11H10N6OS: C, 48.16; H, 3.67; N, 30.64. Found: C, 48.25; H, 3.77; N, 30.83.
Method B: A solution of 7 (0.27 g, 1.5 mmol) and benzyl bromide (0.26 g, 1.5 mmol) in methanolic ammonia (previously saturated at 0°C; 25 mL) was stirred at room temperature in a sealed flask for 24 hours. Evaporation of the solvent on a vacuum evaporator and recrystallization of the residue from water afforded 251 mg (61%) of a material identical in every respect with compound 4 prepared by Method A.
X-ray techniques
X-ray quality crystals of the colorless title compound
4 were obtained by crystallization from dimethyl sulfoxide. Crystal and experimental data are summarized in
Table 2. The data were collected with a NONIUS CAD4 automated diffractometer equipped with a graphite-monochromatized Mo K
α radiation (λ= 0.71073Å) at 295(2) K. The crystal structure data have been deposited at the Cambridge Crystallographic Data Centre [
22]. The unit-cell parameters were determined from 25 reflections with 8.75° ≤ 2
θ ≤ 14.60°. Intensity data with 2
θ ≤ 27.50° were collected with the
ω-2
θ scan technique at 2676 reflections. All reflections were corrected for Lorentz and polarization effects. Absorption corrections were made with the
psi-scan method. The crystal structure was resolved by direct methods using SHELXS-86 [
23] and refined by full-matrix least-squares methods on
F2 using SHELXL-93 [
24]. All non-H atoms were refined anisotropically. Hydrogen atoms were allowed as riding atoms with isotropic displacement parameters related to the non-H atoms on which they were riding.
Table 2.
Crystal and experimental data for compound 4
Table 2.
Crystal and experimental data for compound 4
| Formula | C11H10N6OS |
| Formula weight | 274.31 |
| Crystal system | monoclinic |
| Space group | P21/c |
| Unit-cell dimensions (Å) | a = 7.2926(15) |
| | b = 14.456(2) |
| | c = 11.436(2) |
| | β = 105.30(2)° |
| Unit-cell volume, V (Å3) | 1162.9(4) |
| Formula per unit cell, Z | 4 |
| Dcalcd (g/cm3) | 1.567 |
| Absorption coefficient, μ (mm-1) | 0.28 |
| F(000) | 568 |
| Crystal size (mm) | 0.50 × 0.25 × 0.20 |
| Index ranges | – 9 ≤ h ≤ 9 |
| | 0 ≤ k ≤ 18 |
| | 0 ≤ l ≤ 14 |
| Max. and min. transmission | 0.9769 and 0.8844 |
| Independent reflections | 2676 (Rint = 0.0000) |
| Reflections/restraints/parameters | 2676 /0 /173 |
| Final R indices [I > 2σ(I)] | R1 = 0.0363, wR2 = 0.1041 |
| R indices (all data) | R1 = 0.0482, wR2 = 0.1108 |
| Goodness-of-fit on F2 | 1.062 |
| Max. shift/error | –0.001 |
| Largest difference peak and hole (e/Å3) | 0.377 and – 0.304 |