Synthesis and Analgesic Activity Evaluation of Some Agmatine Derivatives
Abstract
:Introduction
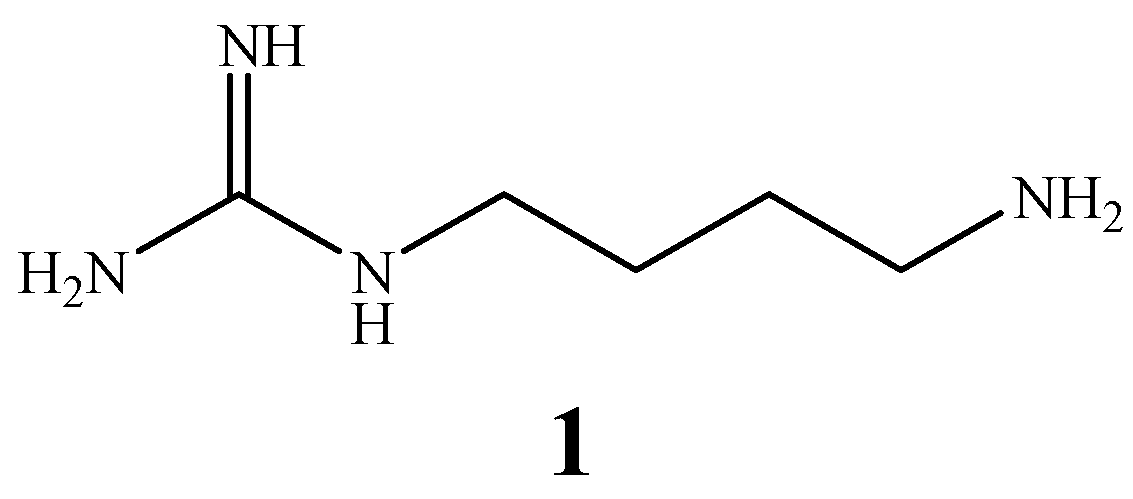
Results and Discussion
Chemistry
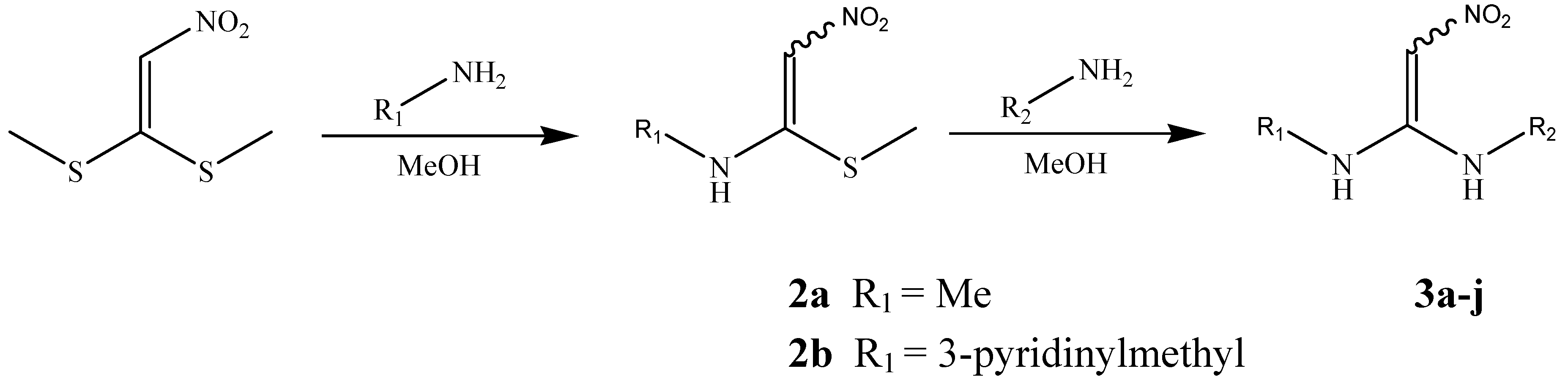
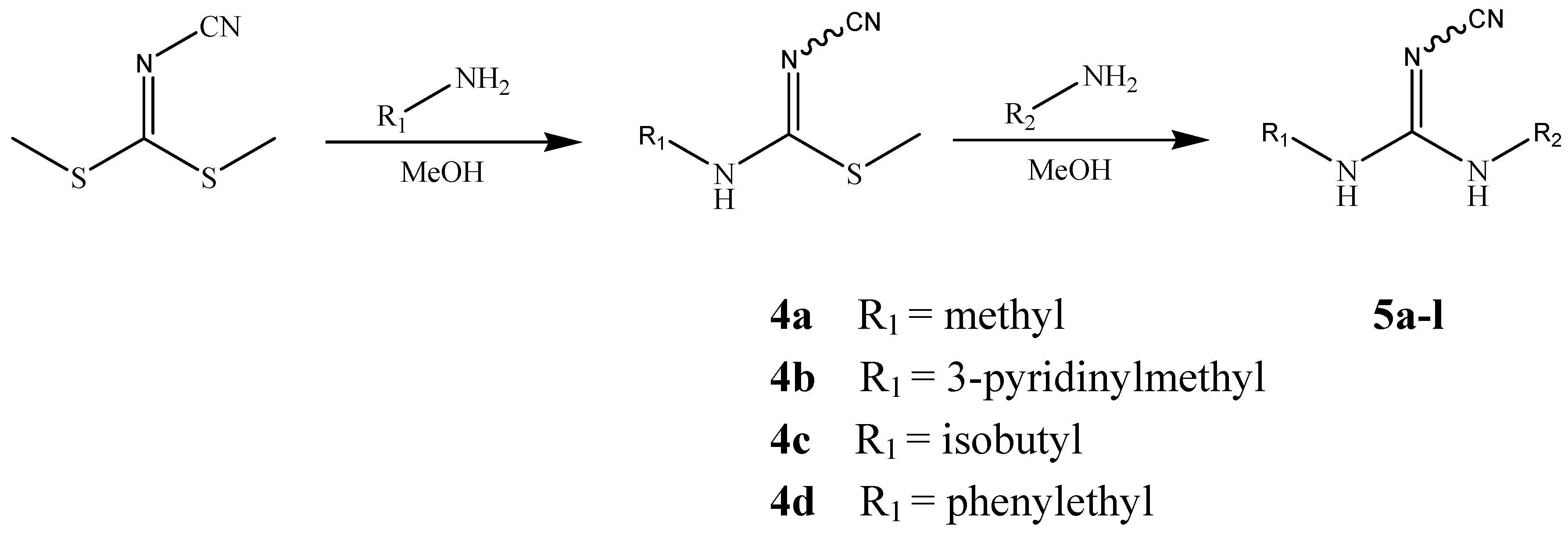
| Compd. | R1 | R2 | Compd. | R1 | R2 |
|---|---|---|---|---|---|
| 3a | CH3 | CH2C (CH3)2CH2N(CH3)2 | 5b | CH3 | (CH2)4NH2 |
| 3b | CH3 | (CH2)4NH2 | 5c | CH3 | (CH2)5NH2 |
| 3c | CH3 | (CH2)5NH2 | 5d | CH3 | (CH2)6NH2 |
| 3d | CH3 | (CH2)6NH2 | 5e | CH3 | (CH2)7NH2 |
| 3e | CH3 | (CH2)4CH3 | 5f | 3-pyridinylmethyl | CH2C(CH3)2CH2N(CH3)2 |
| 3f | CH3 | 4-piperidinobutyl | 5g | 3-pyridinylmethyl | (CH2)6NH2 |
| 3g | CH3 | CH2CCCH2NH2 | 5h | 3-pyridinylmethyl | (CH2)7NH2 |
| 3h | 3-pyridinylmethyl | CH3 | 5i | isobutyl | (CH2)6NH2 |
| 3i | 3-pyridinylmethyl | (CH2)4NH2 | 5j | isobutyl | (CH2)7NH2 |
| 3j | 3-pyridinylmethyl | (CH2)5NH2 | 5k | phenylethyl | (CH2)5NH2 |
| 5a | CH3 | CH2C(CH3)2CH2N(CH3)2 | 5l | phenylethyl | (CH2)7NH2 |
Analgesic activity
Prediction of blood-brain barrier permeation of the compounds
| Compd | inhb.rate (%) | BBB prediction by Volsurf | Compd | inhb.rate (%) | BBB prediction by Volsurf |
|---|---|---|---|---|---|
| Saline | 0 | 5a | 27.0 | 0.79 | |
| Morphine | 100 | 5b | 57.3 | -0.51 | |
| Agmatine | 55.3 | -0.64 | 5c | 19.9 | -0.47 |
| 3a | 35.8* | 0.95 | 5d | 54.1** | -0.34 |
| 3b fumarate | 37.7 | -0.28 | 5e | 53.1 | -0.28 |
| 3c fumarate | 46.5 | -0.18 | 5f fumarate | 56.2* | 0.08 |
| 3d fumarate | 52.0* | -0.12 | 5g fumarate | 37.3 | -0.74 |
| 3e | 33.6* | 0.83 | 5h fumarate | 51.3 | -0.69 |
| 3f | 23.9 | 0.82 | 5i fumarate | 38.4* | -0.28 |
| 3g | 35.4 | -0.35 | 5j fumarate | 24.8 | -0.21 |
| 3h | 60.6* | 0.64 | 5k fumarate | 4.14 | -0.38 |
| 3i fumarate | 47.9 | -0.64 | 5l fumarate | 16.1* | -0.46 |
| 3j fumarate | 27.8 | -0.58 |
Conclusions
Experimental
General
General procedure for the preparation of compounds 2a-b
General procedure for the preparation of compounds 3a-j and their fumarates
General procedure for the preparation of 4a-d
General procedure for the preparation of 5a-l and their fumarates
Analgesic activity
Acknowledgements
References
- Li, G.; Regunathan, S.; Barrow, C. J.; Eshraghi, J.; Copper, R.; Reis, D.J. Agmatine: an endogenous clonidine-displacing substance in the brain. Science 1994, 263, 966–969. [Google Scholar]
- Raasch, W.; Regunathan, S.; Li, G.; Reis, D. J. Agmatine, the bacterial amine, is widely distributed in mammalian tissues. Life Sci. 1995, 56, 2319–2330. [Google Scholar] [CrossRef]
- Kolesnikov, Y.; Jain, S.; Pasternak, G. W. Modulation of opioid analgesia by agmatine. Eur. J. Pharmacol. 1996, 296, 17–22. [Google Scholar] [CrossRef]
- Aricioglu-Kartal, F.; Uzbay, I. T. Inhibitory effect of agmatine on naloxone-precipitated abstinence syndrome in morphine-dependent rats. Life Sci. 1997, 61, 1775–1781. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Pei, G.; Qin, B. Y. Agmatine inhibited tolerance to and dependence on morphine in guinea pig ileum in vitro. Acta. Pharmacol. Sin. 1998, 19, 564–568. [Google Scholar]
- Li, J.; Li, X.; Pei, G.; Qin, B. Y. Effects of agmatine on tolerance to and substance dependence on morphine in mice. Acta. Pharmacol. Sin. 1999, 20, 232–238. [Google Scholar]
- Li, J.; Li, X.; Pei, G.; Qin, B. Y. Analgesic effect of agmatine and its enhancement on morphine analgesia in mice and rats. Acta. Pharmacol. Sin. 1999, 20, 81–85. [Google Scholar]
- Su, R. B.; Li, J.; Qin, B. Y. A biphasic opioid function modulator: agmatine. Acta. Pharmacol. Sin. 2003, 24, 631–636. [Google Scholar]
- Piletz, J. E.; May, P. J.; Wang, G.; Zhu, H. Agmatine Crosses the Blood-Brain Barrier. Ann. N.Y. Acad. Sci. 2003, 1009, 64–74. [Google Scholar] [CrossRef]
- John, C. R.; Brent, M. G.; Kelley, F. K.; Carolyn, A. F. Pharmacodynamic and Pharmacokinetic Studies of Agmatine after Spinal Administration in the Mouse. J. Pharmacol. Exp. Ther. 2005, 314, 1226–1233. [Google Scholar] [CrossRef]
- Perioli, L.; Ambrogi, V.; Bernardini, C.; Grandolini, G.; Ricci, M.; Giovagnoli, S.; Rossi, C. Potential prodrugs of non-steroidal anti-inflammatory agents for targeted drug delivery to the CNS. Eur. J. Med. Chem. 2004, 39, 715–727. [Google Scholar] [CrossRef]
- Masereel, B.; Wouters, J.; Pochet, L.; Lambert, D. Design, Synthesis, and Anticonvulsant Activity of 1-(Pyrid-3-ylsulfonamido)-2-nitroethylenes. J. Med. Chem. 1998, 41, 3239–3244. [Google Scholar] [CrossRef]
- Troschutz, R.; Karger, A. Synthesis of 6-Phenyl-Atevirdine. J. Heterocycl. Chem. 1997, 34, 1147–1151. [Google Scholar] [CrossRef]
- Schou, C.; Ottosen, E. R.; Petersen, H. J.; Bjorkling, F.; Latini, S.; Hjarnaa, P. V.; Bramm, E.; Binderup, L. Novel cyanoguanidines with potent oral antitumour activity. Bioorg. Med. Chem. Lett. 1997, 7, 3095–3100. [Google Scholar] [CrossRef]
- Perez-Medrano, A.; Buckner, S. A.; Coghlan, M. J.; Gregg, R. J.; Gopalakrishnan, M.; Kort, M. E.; Lynch, J. K.; Scott, V. E.; Sullivan, J. P.; Whiteaker, K. L.; Carroll, W. A. Design and synthesis of novel cyanoguanidine ATP-sensitive potassium channel openers for the treatment of overactive bladder. Bioorg. Med. Chem. Lett. 2004, 14, 397–400. [Google Scholar] [CrossRef]
- Falco, J. L.; Lloveras, M.; Buira, I.; Teixido, J.; Borrell, J. I.; Mendez, E.; Terencio, J.; Palomer, A.; Guglietta, A. Design, synthesis and biological activity of acyl substituted 3-amino-5-methyl-1,4,5,7-tetrahydropyrazolo[3,4-b]pyridin-6-ones as potential hypnotic drugs. Eur. J. Med. Chem. 2005, 40, 1179–1187. [Google Scholar] [CrossRef]
- Cruciani, G.; Pastor, M.; Guba, W. VolSurf: a new tool for the pharmacokinetic optimization of lead compounds. Eur. J. Pharm. Sci. 2000, Suppl 2, S29–S39. [Google Scholar] [CrossRef]
- Ooms, F.; Weber, P.; Carrupt, P. A.; Testa, B. A simple model to predict blood-brain barrier permeation from 3D molecular fields. Biochim. Biophys. Acta 2002, 1587, 118–125. [Google Scholar] [CrossRef]
- Rogelio, P. F.; Gregory, P. R. Synthesis and characterization of a novel ranitidine dimmer. Bioorg. Med. Chem. Lett. 1997, 7, 3045–3048. [Google Scholar] [CrossRef]
- Minamida, I.; Iwanaga, K.; Okauchi, T. EP302389; (1989),
- Clitherow, J. W.; Price, B. J.; Bradsaw, J.; Martin-Smith, M. DE 2946332; (1980),
- Minamida, I.; Iwanaga, K.; Tabuchi, T.; Uneme, H.; Danstuji, H.; Okauchi, T. Synthesis and insecticidal activity of acyclic nitroethene compounds containing a 3-pyridylmethylamino group. J. Pestic. Sci. 1993, 18, 31–40. [Google Scholar] [CrossRef]
- Southan, G. J.; Szabo, C.; Thiemermann, C. Isothioureas: potent inhibitors of nitric oxide synthases with variable isoform selectivity. Br. J. Pharmacol. 1995, 114, 510–516. [Google Scholar] [CrossRef]
- Jozesf, R.; Laszlo, P. Synthesis of 1- and 2-R1-3-R2,R3-Amino-5-amino-1,2,4-triazoles. J. Heterocycl. Chem. 1986, 23, 401–408. [Google Scholar] [CrossRef]
- Zhang, H.B.; Huang, H. Y.; Huang, W. L.; Wang, Q. J. Synthesis and antidiabetic activity of sulfonylcyanoguanidines. Zhongguo Yaoke Daxue Xuebao 1999, 30, 243–245. [Google Scholar]
- Sashadri, S.; Sanghavi, N. M.; Naik, R. V.; Tawate, S. R.; Trivedi, M. N.; Fruitwala, M. A. Syntheis and bactericidal activity of some symmetrical bisthioalkyl derivatives. Indian. J. Chem., Sect. B 1993, 32B, 688–692. [Google Scholar]
- Magatti, C. V.; Doll, R. J. US 4803206; (1989),
- Sample availability: Available from the authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
He, H.; Liu, M.; Zheng, Z.; Liu, Y.; Xiao, J.; Su, R.; Hu, C.; Li, J.; Li, S. Synthesis and Analgesic Activity Evaluation of Some Agmatine Derivatives. Molecules 2006, 11, 393-402. https://doi.org/10.3390/11060393
He H, Liu M, Zheng Z, Liu Y, Xiao J, Su R, Hu C, Li J, Li S. Synthesis and Analgesic Activity Evaluation of Some Agmatine Derivatives. Molecules. 2006; 11(6):393-402. https://doi.org/10.3390/11060393
Chicago/Turabian StyleHe, Hongxia, Mengjia Liu, Zhibing Zheng, Ying Liu, Junhai Xiao, Ruibin Su, Chun Hu, Jin Li, and Song Li. 2006. "Synthesis and Analgesic Activity Evaluation of Some Agmatine Derivatives" Molecules 11, no. 6: 393-402. https://doi.org/10.3390/11060393




