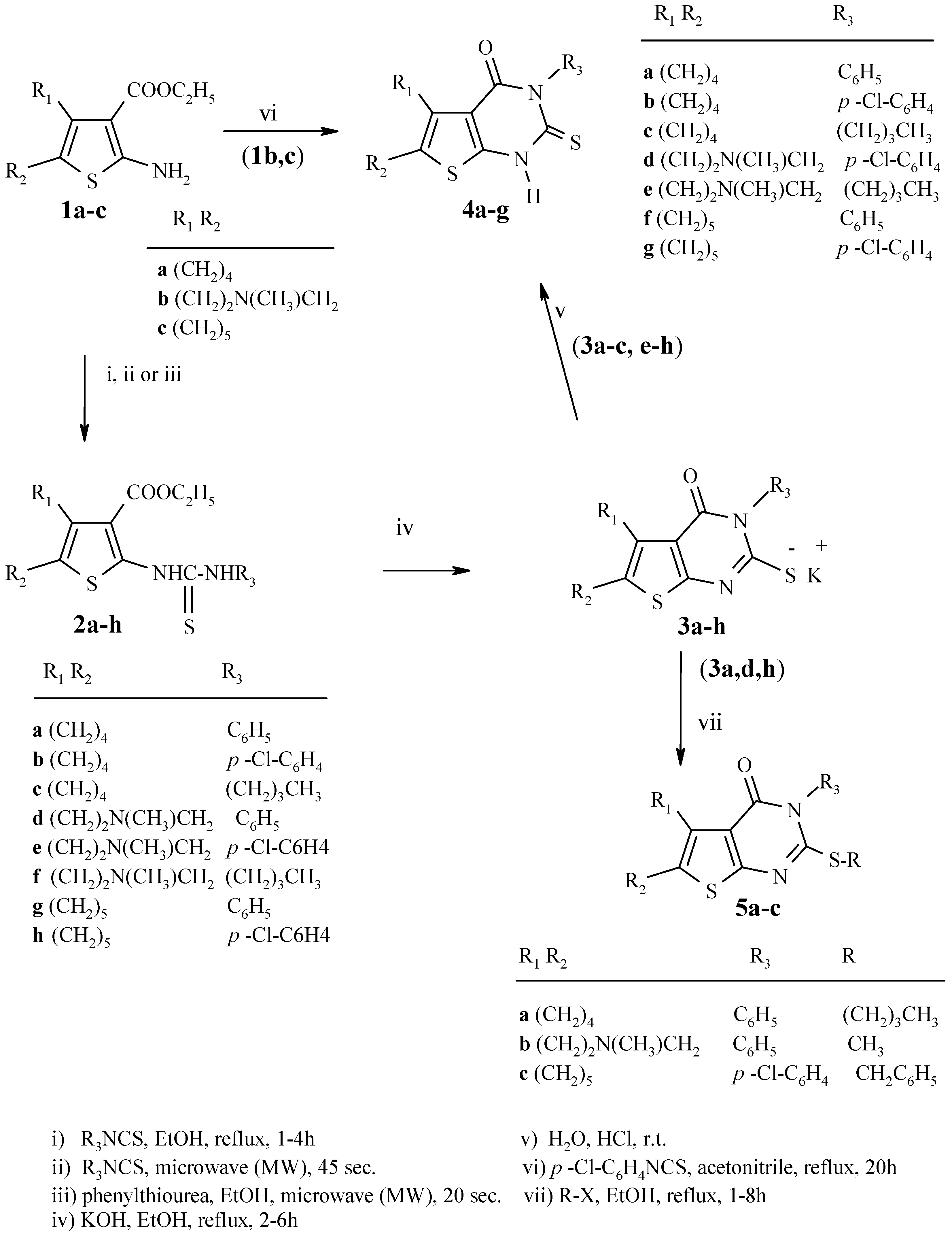
Experimental
General
Melting points were determined using an Electrothermal IA9000 series digital capillary melting point apparatus and are uncorrected. IR spectra were obtained as KBr discs on a 1000-Perkin Elmer FT-IR spectrophotometer.
1H- and
13C-NMR spectra were recorded on a JEOL ECP-400 NMR in CDCl
3 (or DMSO-d
6) using TMS as an internal standard. Chemical shifts are given in ppm on the δ scale and coupling constants (
J) are given in Hz. Electron impact (EI) MS spectra were acquired with the aid of a Shimadzu GCMSQP5050A spectrometer, equipped with a 30 m x 0.25 mm DB-1 glass column, operating with an ionization energy of 70 eV, at the Chemistry Department, College of Science, King Saud University. Compounds
2a-h were synthesized using the reported methods [
8,
9,
13], or in the case of particular examples, by the methods described below.
Method A: Synthesis of 2d:
A mixture of 1b (0.5 g, 2 mmol) and phenyl isothiocyanate (0.27 g, 2 mmol) was placed in a 50 mL beaker covered with a watch glass and then irradiated with microwaves (600 W) for 45 seconds. The cold reaction mixture was treated with ethanol and the solid product was filtered off and recrystallized.
Method B: Synthesis of 2a,d:
A mixture of 1a,b (2 mmol), phenylthiourea (2 mmol) and 5 drops of dry ethanol was placed in a 50 mL beaker, covered with a watch glass, and was then irradiated with microwave (800 W) for 20 seconds. The cold reaction mixture was treated with crushed ice; the solid product was filtered, dried and recrystallized.
3-Ethoxycarbonyl-2-(3-phenylthioureido)-4,5,6,7-tetrahydrobenzo[b]thiophene (2a): Fine pale yellow needles, m.p. 191-193°C (from ethanol); Yield 64%; IR (cm-1): 3416, 3179 (2NH), 1656 (C=O), 1195 (C=S); 1H-NMR (DMSO-d6): 1.30 (3H, t, J = 7.1, CH2CH3), 1.70-1.86 (4H, m, 2CH2 at C-5, C-6), 2.67-2.80 (4H, m, 2CH2 at C-4, C-7), 4.52 (2H, q, J = 7.1, CH2CH3), 7.29-7.33 (2H, m, H-2`, H-6`), 7.52-7.56 (3H, m, H-3`, H-4`, H-5`), 10.21 (1H, br. s, NH), 12.25 (1H, br. s, NH); 13C-NMR: 14.31, 60.75 (Et carbons), 22.09, 23.51, 24.43, 26.42 (aliphatic ring sp3 carbons), 113.02, 121.32, 126.94, 127.11, 130.91, 133.15, 149.85, 135.23 (sp2 carbons), 166.73 (C=O), 176.25 (C=S).
3-Ethoxycarbonyl-2-[3-(4-chlorophenyl)thioureido]-4,5,6,7-tetrahydrobenzo[b]thiophene (2b): Color-less needles, m.p. 221-223°C (from ethanol); Yield 70%; IR (cm-1): 3442, 3196 (2NH), 1663 (C=O), 1198 (C=S); 1H-NMR (DMSO-d6): 1.27 (3H, t, J = 7.3, CH2CH3), 1.68-1.69 (4H, m, 2CH2 at C-5, C-6), 2.51-2.55 (2H, m, CH2 at C-4), 2.67-2.68 (2H, m, CH2 at C-7), 4.18 (2H, q, J = 7.3, CH2CH3), 7.23 (2H, d, J = 8.8, H-2`, H-6`), 7.41 (2H, d, J = 8.8, H-3`, H-5`), 10.53 (1H, br. s, NH), 12.02 (1H, br. s, NH); 13C-NMR: 14.37, 60.49 (Et carbons), 22.97, 23.04, 24.34, 26.43 (aliphatic ring sp3 carbons), 112.23, 126.24, 128.93, 130.58, 137.24 150.42, 161.32, 125.71, (sp2 carbons), 166.66 (C=O), 176.18 (C=S).
2-(3-Butylthioureido)-3-ethoxycarbonyl-4,5,6,7-tetrahydrobenzo[b]thiophene (2c): Fine pale yellow needles, m.p. 123-125°C (from ethanol); Yield 59%; IR (cm-1): 3429, 3230 (2NH), 1655 (C=O), 1175 (C=S); 1H-NMR (CDCl3): 0.93 (3H, t, J = 8.0, CH2CH2CH3), 1.35 (3H, t, J = 7.1, OCH2CH3), 1.40 (2H, sext., J = 8.0, CH2CH2CH3), 1.63 (2H, quint., J = 8.0, CH2CH2CH2), 1.72-1.78 (4H, m, 2CH2 at C-5, C-6), 2.56-2.61 (2H, m, CH2 at C-4), 2.71-2.76 (2H, m, CH2 at C-7), 3.45 (2H, br peak, NHCH2CH2), 4.30 (2H, q, J = 7.1, OCH2CH3), 6.44 (1H, br. s, NH), 12.10 (1H, br. s, NH); 13C-NMR: 14.42, 60.71 (Et carbons), 13.81, 20.11, 30.73, 44.15 (butyl group), 22.92, 23.15, 24.42, 26.51 (aliphatic ring sp3 carbons) 112.01, 126.32, 130.67, 151.43 (thiophene carbons), 167.25 (C=O), 177.03 (C=S).
3-Ethoxycarbonyl-6-methyl-2-(3-phenylthioureido)-4,5,6,7-tetrahydrothieno[2,3-c]pyridine (2d): Fine yellow needles, m.p. 186-188°C (from ethanol/chloroform); Yield 60%; IR (cm-1): 3467, 3175 (2NH), 1658 (C=O), 1195 (C=S); 1H-NMR (CDCl3): 1.24 (3H, t, J = 7.2, CH2CH3), 2.46 (3H, s, CH3N), 2.66 (2H, t, J = 5.9, CH2 at C-4), 2.85 (2H, t, J = 5.8, CH2 at C-5), 3.49 (2H, s, CH2 at C-7), 4.12 (2H, q, J = 7.2, CH2CH3), 7.31-7.36 (3H, m, H-2`,H-4` ,H-6`), 7.47 (2H, t, J =7.4, H-3`,H-5`), 8.01 (1H, br. s, NH), 12.12 (1H, br. s, NH); 13C-NMR: 14.25, 60.56 (Et carbons), 45.63 (CH3N), 52.50, 26.88, 53.24 (aliphatic ring sp3 carbons), 112.54, 124.07, 125.80, 127.95, 129.04, 130.13, 135.81 150.52, (sp2 carbons), 166.14 (C=O), 176.29 (C=S); MS: m/z (%) 375 [M+] (96) (C18H21N3O2S2), 332 [M-CH3-C2H5] (18), 282 [M-C6H5-CH3-H] (13), 239 [M-C6H5NHCS] (100), 166 [M-C6H5NHCS-C2H5OH-C2H4+H] (36).
3-Ethoxycarbonyl-2-[3-(4-chlorophenyl)-6-methylthioureido]-4,5,6,7-tetrahydrothieno[2,3-c]pyridine (2e): Fine yellow cubes, m.p. 206-208°C (from ethanol/ chloroform); Yield 60%; IR (cm-1): 3415, 3180 (2NH), 1659 (C=O), 1195 (C=S); 1H-NMR (CDCl3): 1.27 (3H, t, J = 7.2, CH2CH3), 2.46 (3H, s, CH3N), 2.67 (2H, t, J = 5.7, CH2 at C-4), 2.86 (2H, t, J = 5.7, CH2 at C-5), 3.50 (2H, s, CH2 at C-7), 4.18 (2H, q, J = 7.2, CH2CH3), 7.26 (2H, d, J = 8.8, H-2`, H-6`), 7.41(2H, d, J = 8.8, H-3`, H-5`), 7.89 (1H, br. s, NH), 12.21 (1H, br. s, NH); 13C-NMR: 14.25, 60.78 (Et carbons), 45.63 (CH3N), 26.88, 52.46, 53.21 (aliphatic ring sp3 carbons), 112.46, 123.91, 126.92, 129.04, 130.18, 133.17, 134.53, 150.27 (sp2 carbons), 166.43 (C=O), 176.17 (C=S); MS: m/z (%) 409 [M+] (59) (C18H2035ClN3O2S2), 411 [M+2] (22) (C18H2037ClN3O2S2), 366 [M-CH3-C2H4] (12), 282 [M-ClC6H4-CH3–H] (37), 239 [M-ClC6H4NHCS] (91), 169 [M-ClC6H4NHCS-C2H5OH-C2H4+3H] (58).
2-(3-Butylthioureido)-3-ethoxycarbonyl-6-methyl-4,5,6,7-tetrahydrothieno[2,3-c]pyridine (2f): Fine red needles, m.p. 224-226°C (from ethanol/chloroform);Yield 60%; IR (cm-1): 3434, 3162 (2NH), 1650 (C=O), 1169 (C=S); 1H-NMR (DMSO-d6): 0.89 (3H, t, J = 8.0, CH2CH2CH3), 1.09 (3H, t, J = 7.3, OCH2CH3), 1.31 (2H, sext., J = 7.3, CH2CH2CH3), 1.62 (2H, quint., J = 7.3, CH2CH2CH2), 2.41 (3H, s, CH3N), 2.68 (2H, t, J = 5.9, CH2 at C-4), 2.91-2.95 (2H, m, CH2 at C-5), 3.47 (2H, s, CH2 at C-7), 3.52 (2H, t, J = 7.3, NHCH2CH2), 4.31 (2H, q, J = 7.1, OCH2CH3), 8.85 (1H, br. s, NH), 9.29 (1H, br. s, NH); 13C-NMR: 18.59, 57.36 (Et carbons), 13.93, 20.29, 28.69, 45.98 (Bu carbons), 45.28 (CH3N), 25.47, 51.59, 52.97 (aliphatic ring sp3 carbons), 116.02, 125.17, 129.89, 149.62 (thiophene carbons), 157.19 (C=O), 174.25 (C=S); MS: m/z (%) 355 [M+] (12) (C16H25N3O2S2), 309 [M-C2H5OH] (100), 252 [M-C4H9-C2H5OH] (13), 210 [M-C4H9NHCS-2CH3+H] (35).
General procedure for the preparation of 3a-h:
A mixture of the compounds 2a-h (13.5 mmol) and potassium hydroxide (0.76 g, 13.5 mmol) in absolute ethanol (55 mL) was heated under reflux with stirring for 1 h. The suspension was filtered while hot and the solid was washed with hot absolute ethanol to give 3a-h.
General procedure for the synthesis of 4a-g: Method A:
A suspension of potassium salts of 3a-c,e-h in water (50 mL) was acidified with concentrated hydrochloric acid and stirred at room temperature for 30 min. The solid was collected by filtration, washed with water and recrystallized from ethanol to give 4a-g.
Method B: Synthesis of 4d,g.
A mixture of 1b,c (10 mmol) and the appropriate isothiocyanate (10 mmol) in acetonitrile (30 mL) was heated under reflux for 15 h in the presence of anhydrous potassium carbonate (1.4 g). The reaction mixture was then cooled, filtered, diluted with water (10 mL) and neutralized with 2M hydrochloric acid. The product obtained was filtered, washed with water, dried and recrystallized from ethanol to give 4d,g.
Monopotassium salt of 3-phenyl-2-thioxo-2,3,5,6,7,8-hexahydro-1H-benzo[4,5]thieno[2,3-d]-pyrimidin-4-one (3a) and its 2-thioxo derivative 4a: Yields: 53% (3a) and 73% (4a), respectively; Compound 4a: white powder, m.p. 259-261°C; IR (cm-1): 3413 (NH), 1705 (C=O), 1218 (C=S); 1H-NMR (CDCl3): 1.77-1.85 (4H, m, 2CH2 at C-6, C-7), 2.63-2.68 (2H, m, CH2 at C-5), 2.84-2.91 (2H, m, CH2 at C-8), 7.24-7.26 (2H, m, H-2`, H-6`), 7.45-7.57 (3H, m, H-3`, H-4`, H-5`), 12.19 (1H, br. s, NH); 13C-NMR: 21.96, 22.96, 24.72, 25.14 (aliphatic ring sp3 carbons), 117.53, 128.52, 129.07, 129.49, 129.70, 132.64, 138.56, 148.51 (sp2 carbons), 157.37 (C=O), 174.93 (C=S); MS: m/z (%) 314 [M+] (100) (C16H14N2OS2), 179 [M-C6H5NCS] (89), 151 [M-C6H5NCS-C2H4] (35).
Monopotassium salt of 3-(4-chlorophenyl)-2-thioxo-2,3,5,6,7,8-hexahydro-1H-benzo[4,5]thieno[2,3-d]-pyrimidin-4-one (3b) and its 2-thioxo derivative 4b: Yields: 75% (3b) and 89% (4b), respectively;Compound 4b: white scales, m.p. 289-292°C; IR (cm-1): 3129 (NH), 1706 (C=O), 1219 (C=S); 1H-NMR (DMSO-d6): 1.70-1.71 (2H, m, CH2 at C-6), 1.77-1.78 (2H, m, CH2 at C-7), 2.66-2.70 (2H, m, CH2 at C-5), 2.71-2.75 (2H, m, CH2 at C-8), 7.28 (2H, d, J =8.1, H-2`, H-6`), 7.52 (2H, d, J = 8.1, H-3`, H-5`), 13.71 (1H, br. s, NH); 13C-NMR: 22.10, 23.02, 24.52, 25.38 (aliphatic ring sp3 carbons), 116.67, 128.97, 129.57, 131.62, 131.69, 133.17, 138.81, 149.92 (sp2 carbons), 157.38 (C=O), 174.88 (C=S); MS: m/z (%) 348 [M+] (86) (C16H1335ClN2OS2), 350 [M+2] (35) (C16H1337ClN2OS2), 179 [M-ClC6H4NCS] (100), 151 [M-ClC6H4NCS-C2H4] (38).
Monopotassium salt of 3-butyl-2-thioxo-2,3,5,6,7,8-hexahydro-1H-benzo[4,5]thieno[2,3-d]pyrimidin-4-one (3c) and its 2-thioxo derivative 4c: Yields: 40% (3c) and 78% (4c), respectively; Compound 4c: white scales, m.p. 234-236°C; IR (cm-1): 3254 (NH), 1689 (C=O), 1220 (C=S); 1H-NMR (CDCl3): 0.97 (3H, t, J = 7.3, CH2CH3), 1.42 (2H, sext., J =7.3, CH2CH2CH3), 1.71-1.87 (6H, m, 3CH2 at C-6, C-7, C-2`), 2.66 (2H, t, J = 5.8, CH2 at C-5), 2.91 (2H, t, J = 5.8, CH2 at C-8), 4.43 (2H, t, J = 7.7, NCH2CH2), 12.31 (1H, br. s, NH); 13C-NMR: 13.88, 20.33, 28.82, 46.59, (Bu carbons), 21.99, 22.97, 24.76, 25.31 (aliphatic ring sp3 carbons), 117.31, 129.32 132.29, 147.89 (thiophene carbons), 156.85 (C=O), 173.52 (C=S); MS: m/z (%) 294 [M+] (71) (C16H18N2OS2), 261 [M-SH] (100), 238 [M-C4H9+H] (40), 179 [M-C4H9NCS] (75), 151 [M-C4H9NCS-C2H4] (29).
Monopotassium salt of 7-methyl-3-phenyl-2-thioxo-2,3,5,6,7,8-hexahydro-1H-pyrido[3`,4`:5,4]-thieno[2,3-d]pyrimidin-4-one (3d): Yield 45%; IR (cm-1): 1650 (C=O); 1H-NMR (DMSO-d6): 2.31 (3H, s, CH3N), 3.21 (2H, t, J = 5.9, CH2 at C-5), 3.38-3.42 (2H, m, CH2 at C-6), 4.33 (2H, s, CH2 at C-8), 7.31-7.33 (3H, m, H-2`, H-4`, H-6`), 7.45-7.52 (2H, t, J =7.8, H-3`, H-5`); 13C-NMR: 46.44 (CH3N), 26.24, 53.50, 54.31 (aliphatic ring sp3 carbons), 118.79,122.37, 128.37, 129.83, 131.45, 131.65, 142.13, 157.74, (sp2 carbons), 165.68 (C=O), 176.23 (C=S).
Monopotassium salt of 3-(4-chloro-phenyl)-7-methyl-2-thioxo-2,3,5,6,7,8-hexahydro-1H-pyrido-[3`,4`:5,4]thieno[2,3-d]pyrimidin-4-one (3e) and its 2-thioxo derivative 4d: Yields: 85% (3e) and 89% (4d), respectively; Compound 4d: pale orange scales, m.p. 256-258°C; IR (cm-1): 3137 (NH), 1691 (C=O), 1213 (C=S); 1H-NMR (DMSO-d6): 2.88 (3H, s, CH3N), 2.98-3.12 (2H, m, CH2 at C-5), 3.36-3.46 (2H, m, CH2 at C-6), 4.39 (2H, s, CH2 at C-8), 7.29 (2H, d, J = 8.1, H-2`, H-6`), 7.53 (2H, d, J = 8.1, H-3`, H-5`), 11.18 (1H, br. s, NH); 13C-NMR: 42.22 (CH3N), 22.79, 49.89, 50.55 (aliphatic ring sp3 carbons), 115.56, 120.55, 128.83, 129.64, 131.56, 133.31, 138.57, 151.80, (sp2 carbons), 157.32 (C=O), 175.38 (C=S); MS: m/z (%) 363 [M+] (84) (C16H1435ClN3OS2), 365 [M+2] (35) (C16H1437ClN3OS2), 193 [M-ClC6H4NCS] (21), 151 [M-ClC6H4NCS-C2H4-CH3] (100).
Monopotassium salt of 7-methyl-3-butyl-2-thioxo-2,3,5,6,7,8-hexahydro-1H-pyrido[3`,4`:5,4]thieno-[2,3-d]pyrimidin-4-one (3f) and its 2-thioxo derivative 4e: Yields: 51% (3f) and 45% (4e), respectively; Compound 4e: yellow scales, m.p. 297-299°C; IR (cm-1): 3176 (NH), 1687 (C=O), 1200 (C=S); 1H-NMR (DMSO-d6): 0.92 (3H, t, J = 7.3, CH2CH3), 1.32 (2H, sext., J =7.3, CH2CH2CH3), 1.62 (2H, quint., J =7.3, CH2CH2CH2),4.41 (2H, t, J = 7.7 NCH2CH2 ), 2.49 (3H, s, CH3N), 2.84-2.89 (2H, m, CH2 at C-5), 3.14-3.20 (2H, m, CH2 at C-6 ), 3.34 (2H s,, CH2 at C-8), 13.71 (1H, br. s, NH); 13C-NMR: 14.47, 20.25, 28.69, 45.93 (Bu carbons), 45.42 (CH3N), 26.81, 52.54, 53.22 (aliphatic ring sp3 carbons), 114.42, 124.98, 130.76, 149.81 (thiophene carbons), 156.38 (C=O), 175.94 (C=S); MS: m/z (%) 309 [M+] (100) (C14H19N3OS2), 193 [M-C4H9NCS-H] (15), 151 [M-C4H9NCS-C2H4-CH3] (14).
Monopotassium salt of 3-phenyl-2-thioxo-1,2,3,5,6,7,8,9-octahydrocyclohepta[4,5]thieno[2,3-d]-pyrimidin-4-one (3g) and its 2-thioxo derivative 4f: Yields: 42% (3g) and 83% (4f); Compound 4f: pale yellow powder, m.p. 306-308°C; IR (cm-1): 3141 (NH), 1698 (C=O), 1221 (C=S); 1H-NMR (DMSO-d6): 1.54-1.62 (2CH2 m, 4H at C-6, C-7), 1.81 (2H, br. peak, CH2 at C-8), 2.76 (2H, br. peak, CH2 at C-5), 3.09 (2H, br. peak, CH2 at C-9), 7.20-7.21 (2H, d, J = 7.1, H-2`, H-6`), 7.38 (1H, t, J = 7.3, H-4`), 7.45 (2H, t, J = 7.3, H-3`, H-5`), 13.57 (1H, br. s, NH); 13C-NMR: 27.17 (2C), 27.86, 28.95, 32.26 (aliphatic ring sp3 carbons), 117.23, 128.47, 129.38, 129.58, 132.66, 137.20, 139.99, 148.57 (sp2 carbons), 157.94 (C=O), 174.87 (C=S); MS: m/z (%) 328 [M+] (100) (C17H16N2OS2), 193 [M-C6H5NCS] (39), 151 [M-C6H5NCS-C2H4-NH+H] (6).
Monopotassium salt of 3-(4-chlorophenyl)-2-thioxo-1,2,3,5,6,7,8,9-octahydrocyclohepta[4,5]thieno-[2,3-d]pyrimidin-4-one (3h) and its 2-thioxo derivative 4g: Yields: 88% (3h) and 85% (4g), respectively; Compound 4g: white scales, m.p. 263-265°C, 3123 (NH), 1710 (C=O), 1220 (C=S); 1H-NMR (DMSO-d6): 1.53 (2H, br. peak, CH2 at C-7), 1.61 (2H, br. peak, CH2 at C-6), 1.82 (2H, br. peak, CH2 at C-8), 2.77 (2H, br. peak, CH2 at C-5), 3.08 (2H, br. peak, CH2 at C-9), 7.28 (2H, d, J = 8.8, H-2`,H-6`), 7.52 (2H, d, J = 8.8, H-3`,H-5`), 13.68 (1H, br. s, NH); 13C-NMR: 27.14 (2C), 27.86, 28.93, 32.33 (aliphatic ring sp3 carbons), 117.20, 129.52, 131.65, 132.79, 133.12, 137.21, 138.95, 148.63 (sp2 carbons), 157.90 (C=O), 174.62 (C=S); MS: m/z (%) 362 [M+] (100) (C17H1535ClN2OS2), 364 [M+2] (44) (C17H1537ClN2OS2), 193 [M-ClC6H4NCS] (74), 151 [M-ClC6H4NCS-C2H4-NH+H] (19).
General procedure for synthesis of 5a-c:
A mixture of potassium salt of 3a, d, h (3.6 mmol) and the appropriate alkyl halide (4.32 mmol) in ethanol (15 mL) was heated under reflux with stirring for 1 h (for compounds 5a,b) and for 8 h (for compound 5c). The solid obtained was filtered, washed with water, dried and recrystallized from ethanol/chloroform.
2-Butylthio-3-phenyl-5,6,7,8-tetrahydro-3H-benzo[4,5]thieno[2,3-d]pyrimidin-4-one (5a): Colorless scales, m.p. 230-232°C; Yield 90%; IR (cm-1): 1689 (C=O); 1H-NMR (CDCl3): 0.90 (3H, t, J = 7.3, CH2CH3), 1.39 (sext., 2H, J =7.3, CH2CH2CH3), 1.61 (2H, quint., J =7.3, CH2CH2CH2), 1.79-1.87 (4H, m, 2CH2 at C-6, C-7), 2.70-2.76 (2H, m, CH2 at C-5), 2.91-2.94 (m, 2H, CH2 at C-8), 3.12 (2H, t, J = 7.3, NCH2CH2), 7.25-7.27 (2H, m, H-2`, H-6`), 7.51-7.53 (3H, m, H-3`, H-4`, H-5`); 13C-NMR: 13.73, 22.04, 30.59, 32.59 (Bu carbons), 22.38, 23.10, 25.17, 25.46 (aliphatic ring sp3 carbons), 119.15, 129.12, 129.76, 129.89, 131.68, 131.74, 136.04, 157.75 (sp2 carbons), 158.69 (C=O), 162.11 (C=S); MS: m/z (%) 370 [M+] (100) (C20H22N2OS2), 314 [M-C4H9+H] (74), 281 [M-SC4H9] (39), 253 [M-SC4H9-C2H4] (6), 179 [M-SC4H9-C2H4-C6H5+3H] (79), 151 [M-C4H9-C2H4-C6H5NCS+H] (16).
3-Phenyl-7-methyl-2-methylthio-5,6,7,8-tetrahydro-3H–pyrido[3`,4`:5,4]thieno[2,3-d]pyrimidin-4-one (5b): Fine yellow needles, m.p. 296-298°C; Yield 75%; IR (cm-1): 1692 (C=O); 1H-NMR (DMSO-d6): 2.45 (3H, s, NCH3), 3.17-3.21 (2H, m, CH2 at C-5), 3.36 (3H, s, SCH3), 3.71 (2H, t, J = 5.9, CH2 at C-6), 4.75 (2H, s, CH2 at C-8), 7.41-7.42 (2H, m, H-2`, H-6`), 7.55-7.62 (3H, m, H-3`, H-4`, H-5`); 13C-NMR: 15.93 (SCH3), 51.44 (CH3N), 22.20, 58.51, 60.01 (aliphatic ring sp3 carbons), 117.88, 121.26, 127.43, 129.71, 130.24, 130.63, , 136.02, 157.84 (sp2 carbons), 161.18 (C=O), 163.85 (C=S); MS: m/z (%) 343 [M+] (88) (C17H17N3OS2), 328 [M-CH3] (47), 300 [M-CH3-C2H4] (100), 253 [M-CH3-C2H4-SCH3] (83), 193 [M-CH3-C6H5NCS] (6), 150 [M-2CH3-C6H5NCS-C2H4] (27).
2-Benzylthio-3-(4-chloro-phenyl)3,5,6,7,8,9-hexahydrocyclohepta[4,5]thieno[2,3-d]pyrimidin-4-one (5c): Fine colorless needles, m.p. 264-266°C; Yield 88%; IR (cm-1): 1680 (C=O); 1H-NMR (CDCl3): 1.61-1.74 (4H, m, 2CH2 at C-6, C-7 ), 1.84-1.88 (2H, m, CH2 at C-8), 2.82-2.85 (2H, m, CH2 at C-5), 3.23-3.26(2H, m, CH2 at C-9), 4.33 (2H, s, SCH2Ph), 7.19 (2H, d, J = 8.8, H-2`, H-6`), 7.23-7.34 (5H, m, Ar-H), 7.46 (d, 2H, J = 8.8, H-3`, H-5`); 13C-NMR: 27.32, 27.78, 27.90, 29.94, 32.69 (aliphatic ring sp3 carbons), 37.31 (SCH2Ph), 119.70, 127.71, 128.68, 129.44, 130.06, 130.60, 134.33, 135.91, 136.06, 136.60, 137.37, 156.14 (sp2 carbons), 159.04 (C=O), 160.90 (C=S); MS: m/z (%) 452 [M+] (67) (C24H2135ClN2OS2), 454 [M+2] (30) (C24H2137ClN2OS2), 419 [M-Cl+2H] (29), 361 [M-CH2C6H5] (6), 326 [M-Cl-CH2C6H5] (9), 201 [M-SHCH2C6H5-ClC6H4NH2] (100).
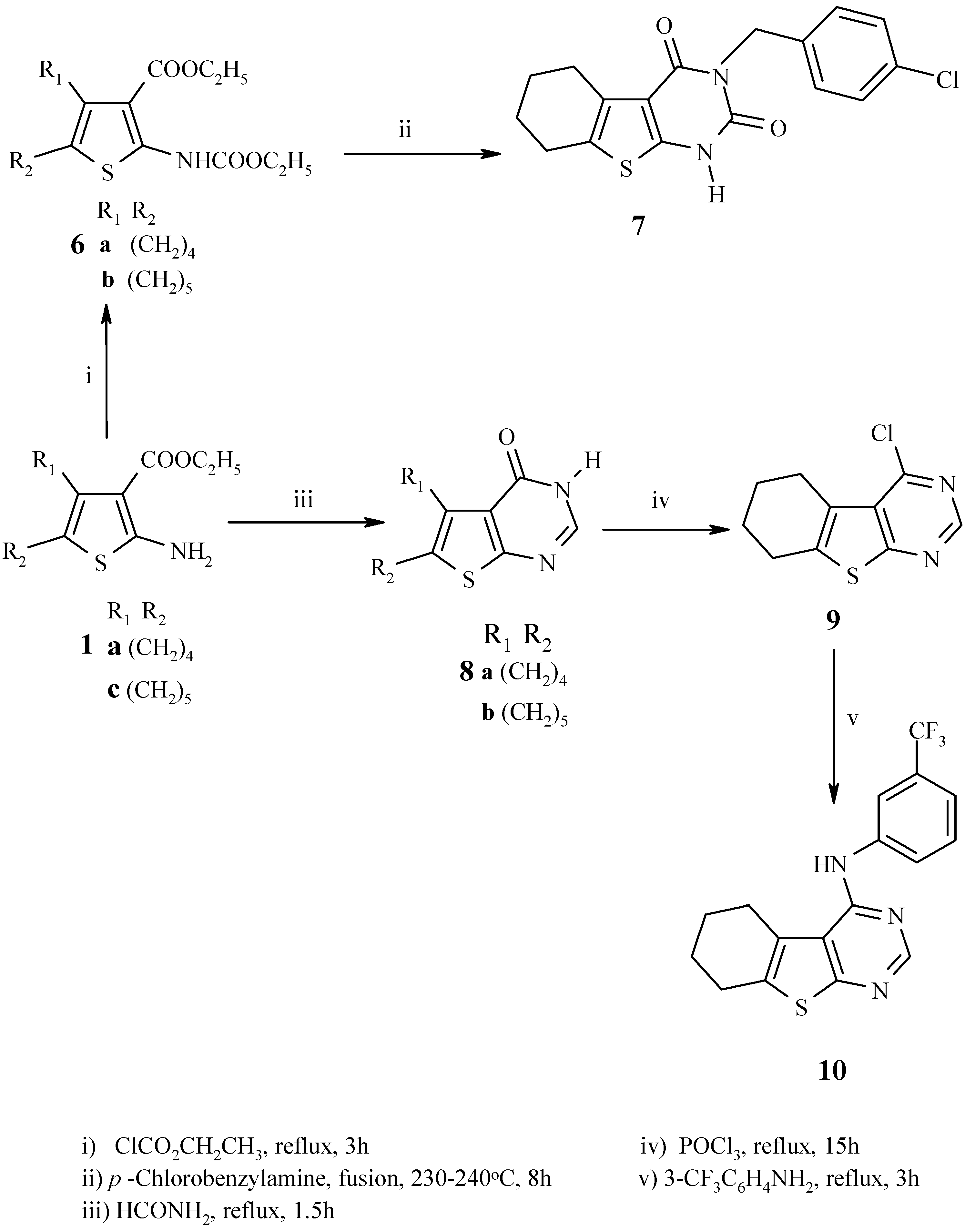
General procedure for synthesis of 6a,b:
A mixture of 1a,c (15.8 mmol) and ethyl chloroformate (40 mL) was refluxed for 3h. After cooling, the reaction mixture was evaporated under reduced pressure and the residue was recrystallized from ethanol.
2-Ethoxycarbonylamino-3-ethoxycarbonyl-5,6,7,8-tetrahydrobenzo[b]thiophene (6a): Fine pale brown needles, m.p. 66-68 °C; Yield 87%; IR (cm-1): 3246 (NH), 1724, 1662 (2C=O); 1H-NMR (DMSO-d6): 1.25 (3H, t, J = 7.3 CH2CH3), 1.28 (3H, t, J = 6.9 CH2CH3), 1.68-1.70 (4H, m, 2CH2 at C-5, C-6), 2.54-2.56 (2H, m, CH2 at C-4), 2.64-2.66 (2H, m, CH2 at C-7), 4.19 (2H, q, J = 7.3 OCH2CH3), 4.23 (2H, q, J = 7.3 OCH2CH3), 10.34 (1H, br s, NH); 13C-NMR: 14.56, 62.59 (Et carbons), 14.78, 60.89 (Et carbons), 22.77, 23.01, 24.27, 26.42 (aliphatic ring sp3 carbons), 111.01, 125.69, 131.32, 148.62 (thiophene carbons), 165.85 (C=O), 152.75 (carbamate C=O); MS: m/z (%) 297 [M+] (100) (C14H19NO4S), 251 [M-C2H5OH] (89), 223 [M-C2H5COOH] (30), 205 [M-2C2H5OH] (38), 195 [M-C2H5COOH-C2H4] (10), 179 [M-COOC2H5-C2H5O] (54), 151 [M-COOC2H5-C2H5O-C2H4] (32).
2-Ethoxycarbonylamino-3-ethoxycarbonyl-5,6,7,8-tetrahydro-4H-cyclohepta[b]thiophene (6b): Fine pale brown needles, m.p. 78-80 °C; Yield 70%; IR (cm-1): 3241 (NH), 1728, 1662 (2C=O); 1H-NMR (DMSO-d6): 1.25 (3H, t, J = 6.9 CH2CH3), 1.28 (3H, t, J = 7.1, CH2CH3), 1.52-1.58 (4H, m, 2CH2 at C-5, C-6), 1.76-1.78 (2H, m, CH2 at C-7), 2.65-2.69 (2H, m, CH2 at C-4), 2.90-2.93 (2H, m, CH2 at C-8), 4.18 (2H, q, J = 7.1 OCH2CH3), 4.26 (2H, q, J = 7.4 OCH2CH3), 10.11 (1H, br. s, NH); 13C-NMR: 14.82, 62.40 (Et carbons), 14.53, 61.05 (Et carbons), 27.12, 27.98, 28.14, 28.45, 32.11 (aliphatic ring sp3 carbons), 116.25, 130.13, 137.03, 145.61 (thiophene carbons), 165.55, (C=O), 153.04 (carbamate C=O); MS: m/z (%) 311 [M+] (100) (C15H21NO4S), 283 [M-C2H4] (8), 265 [M-C2H5OH] (72), 237 [M-HCOOC2H5] (26), 219 [M-HCOOC2H5-CH3-3H] (35), 193 [M-COOC2H5-C2H5O] (33), 165 [M-COOC2H5-C2H5O-C2H4] (17), 151 [M-COOC2H5-C2H5O-C2H4-CH3+H] (7), 139 [M-COOC2H5-C2H5O-C2H4-HCN+H] (10).
3-(4-Chlorobenzyl)-5,6,7,8-tetrahydro-1H-benzo[4,5]thieno[2,3-d]pyrimidine-2,4-dione (7):
A mixture of 6a (1.2 g, 3.7 mmol) and 4-chlorobenzylamine (1.0 g, 7.1 mmol) was heated to 230-240 °C for 8 h. After cooling, the crude solid was recrystallized from ethanol/water to give 7 as a brown powder, m.p.236-238 °C; Yield 60%; IR (cm-1): 3232 (NH), 1724 (C=O at 4 position), 1660 (C=O at 2 position); 1H-NMR (CDCl3): 1.78-1.85 (4H, m, 2 CH2 at C-6, C-7) 2.61-2.63 (2H, m, CH2 at C-5) 2.87-2.89 (2H, m, CH2 at C-8),5.09 (2H, s, NCH2C6H4Cl), 7.23 (2H, d, J = 8.8, H-2`, H-6`),7.41 (2H, d, J = 8.8, H-3`, H-5`), 10.27 (1H, br. s, NH); 13C-NMR: 22.07, 23.15, 24.59, 25.45 (aliphatic ring sp3 carbons), 43.13 (NCH2 Ar), 113.91, 126.98, 128.57, 130.44, 132.34, 133.84, 135.58, 148.52, (sp2 carbons), 152.41 (C=O at C-2), 158.90 (C=O at C-4); Ms: m/z (%) 346 [M+] (41) (C17H1537ClN2O2S), 348 [M+2] (17) (C17H1535ClN2O2S), 179 (30) [M-OCNCH2C6H4Cl], 308 (22) [M-HCl-2H], 221 (61) [M-CH2C6H4Cl], 151 (17) [M-OCNCH2C6H4Cl-CO], 140 (98) [M-C10H8NO2S], 125 (100) [M-C10H9N2O2S].
General procedure for synthesis of 8a,b:
A mixture of 1a,c (2 mmol) and formamide (20 mL) was heated under reflux for 1.5 h, then left to cool to room temperature overnight. The solid formed was filtered, washed with water, dried and recrystallized from ethanol.
5,6,7,8-Tetrahydro-3H-benzo[4,5]thieno[2,3-d]pyrimidin-4-one (8a): Fine pale brown needles, m.p. 255-257 °C; Yield 92%; IR (cm-1): 3415 (NH), 1693 (C=O); 1H-NMR (DMSO-d6): 1.73-1.79 (4H, m, 2CH2 at C-6, C-7), 2.71-2.74 (2H, m, CH2 at C-5), 2.85-2.87 (2H, m, CH2 at C-8), 7.99 (1H, s, H-2), 12.29 (1H, br. s, NH); 13C-NMR: 22.32, 23.01, 24.99, 25.89 (aliphatic ring sp3 carbons), 123.23, 131.36, 136.77, 145.39 (thiophene carbons), 158.26 (C-2), 162.97 (C=O); Ms: m/z (%) 206 [M+] (100) (C10H10N2OS), 191 [M-NH] (36), 178 [M-CO] (91), 165 [M-CONH+2H] (7), 150 [M-CO-C2H4] (7), 136 [M-CONH-HCN] (3).
3,5,6,7,8,9-Hexahydrocyclohepta[4,5]thieno[2,3-d]pyrimidin-4-one (8b): Fine pale brown needles, m.p. 209-211 °C; Yield 90%; IR (cm-1): 3411 (NH), 1705 (C=O); 1H-NMR (DMSO-d6): 1.53-1.65 (4H, m, 2CH2 at C-6, C-7), 1.80-1.87 (2H, m, CH2 at C-8), 2.80-2.83 (2H, m, CH2 at C-5), 3.23-3.26 (2H, m, CH2 at C-9) 7.98 (1H, s, H-2), 12.31 (1H, br. s, NH); 13C-NMR: 27.42, 27.80, 27.86, 29.57, 32.56 (aliphatic ring sp3 carbons), 123.76, 132.15, 137.01,145.07 (thiophene carbons), 158.75 (C-2), 161.39 (C=O); MS: m/z (%) 220 [M+] (100) (C11H12N2OS), 205 [M-NH] (67), 191 [M-CHO] (46), 192 [M-CO] (48), 178 [M-CONH+H] (23), 165 [M-CO-C2H4+H] (25), 148 [M-HCONH2-HCN] (6).
4-Chloro-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine (9):
A mixture of 8a (1 g, 5 mmol) and phosphorus oxychloride (10 mL) was heated under reflux for 15 h. The excess phosphorus oxychloride was removed by distillation under reduced pressure, the residue treated with dry benzene (5 mL) and the solvent distilled under reduced pressure to remove the last traces of phosphorus oxychloride. The residue left was triturated with ice and sodium bicarbonate solution (10 %), the solid thus obtained was collected, washed with water and recrystallized from toluene to give a 48% yield of the title compound 9 as fine pale brown needles, m.p. 90-92 °C; 1H-NMR (CDCl3): 1.89-1.91 (4H, m, 2CH2 at C-6, C-7), 2.86-2.87 (2H, m, CH2 at C-5), 3.05-3.06 (2H, m, CH2 at C-8), 8.68 (1H, s, H-2); 13CNMR: 22.27, 22.50, 26.12, 26.36 (aliphatic ring sp3 carbons), 127.23, 128.91, 139.72, 153.23, 168.89, 151.57 (sp2 carbons); Ms: m/z (%) 224 [M+] (65) (C10H935ClN2S); 226 [M+2] (25) (C10H937ClN2S), 196 (100) [M-C2H4], 161 (10) [M-HCN-HCl].
4-N-(3-Trifluoromethylphenyl)-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine (10):
A mixture of 9 (0.5 g, 2 mmol) and 3-trifluoromethyl aniline (5 g, 40 mmol) was heated under reflux for 3 h and left overnight. The oily product was treated several times with petroleum ether (b.p. 40-60°C) and the separated solid was washed several times with petroleum ether to give a 15% yield of compound 10 as a brown powder, m.p. 224-226 °C; IR (cm-1): 3450 (NH); 1H-NMR (CDCl3): 1.91-2.01 (4H, m, 2CH2 at C-6, C-7), 2.84-2.86 (2H, m, CH2 at C-5), 3.06-3.07 (2H, m, CH2 at C-8), 7.29 (1H, s, H-2`), 7.35 (1H, d, J = 8.1, H-6`), 7.47 (1H, t, J = 7.6, H-5`), 7.89 (1H, d, J = 8.1 , H-4`), 7.95 (1H, br. s, NH); 13C-NMR: 22.41, 22.55, 25.62, 26.58 (aliphatic ring sp3 carbons), 120.51 (CF3), 116.93, 117.69, 117.73, 120.47, 124.22, 124.67, 129.62, 135.78, 139.03, 151.82,, 154.67, 165.66 (sp2 carbons); MS: m/z (%) 349 [M+] (100) (C17H14F3N3S), 334 [M-F+4H] (20), 320 [M-C2H4-H] (10), 304 [M-C2H4-F+2H] (5), 294 [M-3F+2H] (2), 204 [M-C6H4CF3] (16).
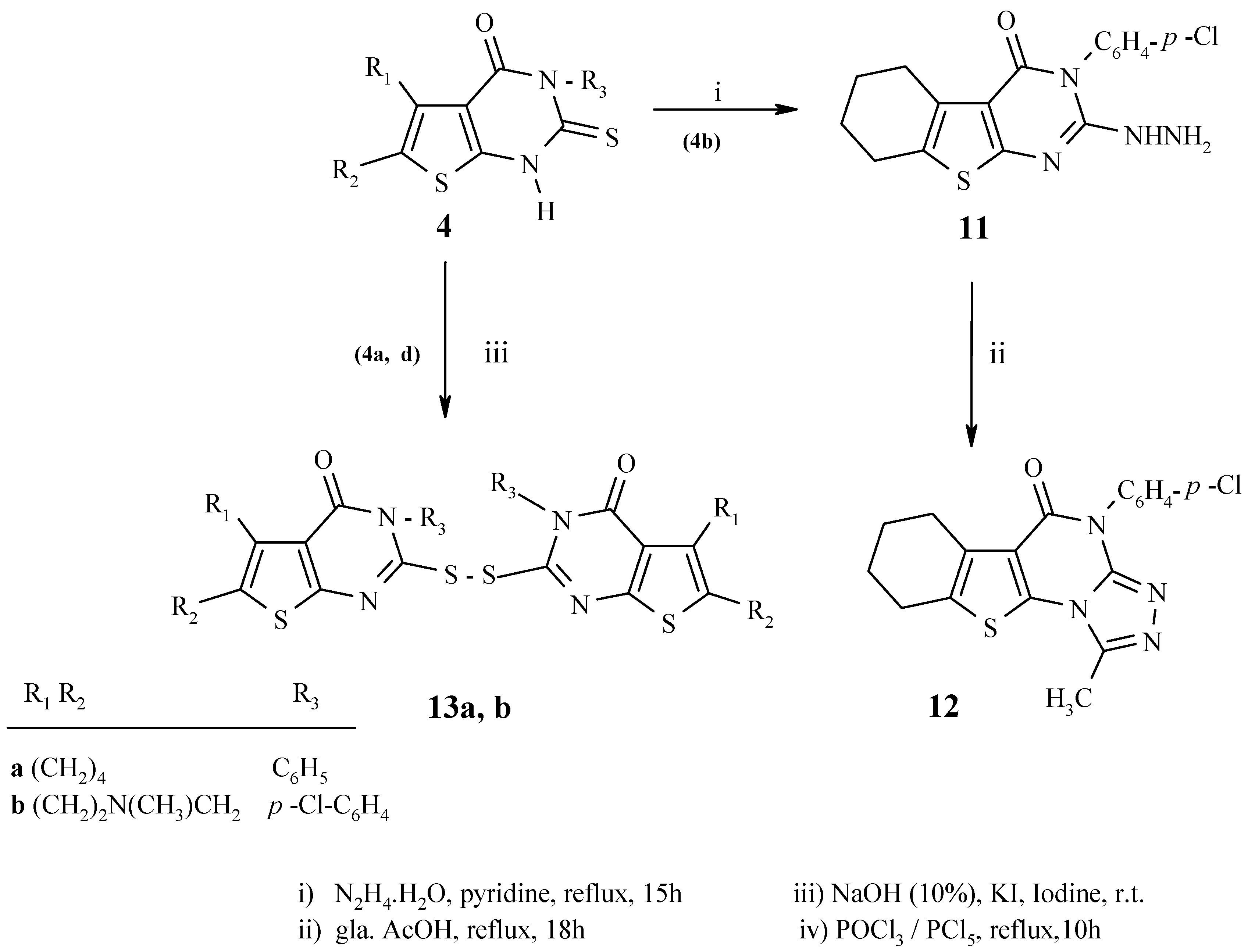
3-(4-Chlorophenyl)-2-hydrazino-5,6,7,8-tetrahydro-3H-benzo[4,5]thieno[2,3-d]pyrimidin-4-one (11):
A mixture of 4b (1.4 g, 4 mmol) and 99% hydrazine hydrate (4 mL, 80 mmol) in pyridine (20 mL) was heated under reflux for 15 h. The mixture was evaporated under reduced pressure and the residue was treated with ethanol. The solid product was filtered and washed several times with ethanol to give a 75% yield of 11 as colorless needles, m.p. 204-204 °C; IR (cm-1): 3488-3272 (NH, NH2); 1H-NMR (acetic acid-d4): 1.74-1.86 (4H, m, 2CH2 at C-6, C-7), 2.67-2.68 (2H, m, CH2 at C-5), 2.81-2.83 (2H, m, CH2 at C-8), 7.38 (1H, br. s, NH), 7.39 (1H, br. s, NH), 7.56 (1H, br. s, NH), 7.43 (2H, d, J = 8.7, H-2`, H-6`), 7.58 (2H, d, J = 8.7, H-3`, H-5`); MS: m/z (%) 346 [M+] (89) (C16H1535ClN4OS), 348 [M+2] (34) C16H1537ClN4OS), 331 [M-NH2+H] (100), 316 [M-N2H4+2H] (32), 303 [M-NH2+H-C2H4] (63), 220 [M-NH-C6H4Cl] (22).
4-(4-Chlorophenyl)-1-methyl-6,7,8,9-tetrahydro-4H-benzo[4,5]thieno[2,3-d][1,2,4]triazolo[3,4-b]-pyrimidin-5-one (12):
A mixture of 11 (1.4 g, 4 mmol) and glacial acetic acid (15 mL) was heated under reflux with stirring for 8 h. The reaction mixture was allowed to cool to room temperature and was poured into water (50 mL). The formed solid was collected by filtration, washed with ethanol, dried and recrystallized from ethanol to give 12 as colorless fine needles, m.p. 300-300 °C; Yield 72%; IR (cm-1): 1676 (C=O); 1H-NMR (CDCl3): 1.83-1.91 (4H, m, 2CH2 at C-7, C-8), 2.79- 2.81 (5H, m, CH3, CH2 at C-6), 3.00 (2H, t, J = 6.2, CH2 at C-9), 7.37 (2H, d, J = 8.8, H-2`, H-6`), 7.51 (2H, d, J = 8.8, H-3`, H-5`); MS: m/z (%) 370 [M+] (100) (C18H1535ClN4OS), 372 [M+2] (38) C18H1537ClN4OS), 355 [M-CH3] (4), 328 [M-CH3-C2H4+H] (22).
General procedure for synthesis of 13a,b:
A solution of iodine (50.76 g, 20 mmol), in 5% KI solution (100 mL) was added dropwise with stirring to a solution of 4a,d (10 mmol) in 10% aqueous sodium hydroxide (10 mL) until the color of iodine persisted. The solid formed was filtered and dried.
Bis{3-phenyl-5,6,7,8-tetrahydro-3H-benzo[4,5]thieno[2,3-d]pyrimidin-4-on-2-yl}disulfide (13a): Pale yellow cubes, m.p. 277-279 °C (from toluene/chloroform); Yield 73%; IR (cm-1): 1651 (C=O); 1H-NMR (CDCl3): 1.79-1.87 (8H, m, 4CH2 at C-6, C-7), 2.74-2.77 (4H, m, 2CH2 at C-5), 2.91-2.95 (4H, m, 2CH2 at C-8), 7.39-7.42 (4H, m, H-2`, H-6`), 7.55-7.63 (6H, m, H-3`, H-4`, H-5`), 7.39-7.42 (4H, m, H-2`, H-6`); 13C-NMR: 22.30, 23.01, 25.24, 25.35 (aliphatic ring sp3 carbons), 119.98, 129.40, 130.11, 130.60, 131.87, 133.21, 135.28, 153.09, 161.92 (sp2 carbons); 158.42 (C=O); (C-2); MS: m/z (%) 626 [M+] (0.4) (C32H26N4O2S4); 314 (100) [M-C16H13N2OS2]; 281 [M-C16H13N2OS2-SH+H] (19).
Bis{3-(4-Chlorophenyl)-7-methyl-5,6,7,8-tetrahydro-3H–pyrido[3`,4`:5,4]thieno[2,3-d]pyrimidin-4-on-2-yl}disulfide (13b): Yield 65%; m.p. 254-256 °C; IR (cm-1): 1651 (C=O); MS: m/z (%) 363 (91) [M-C16H13ClN3OS2+H].
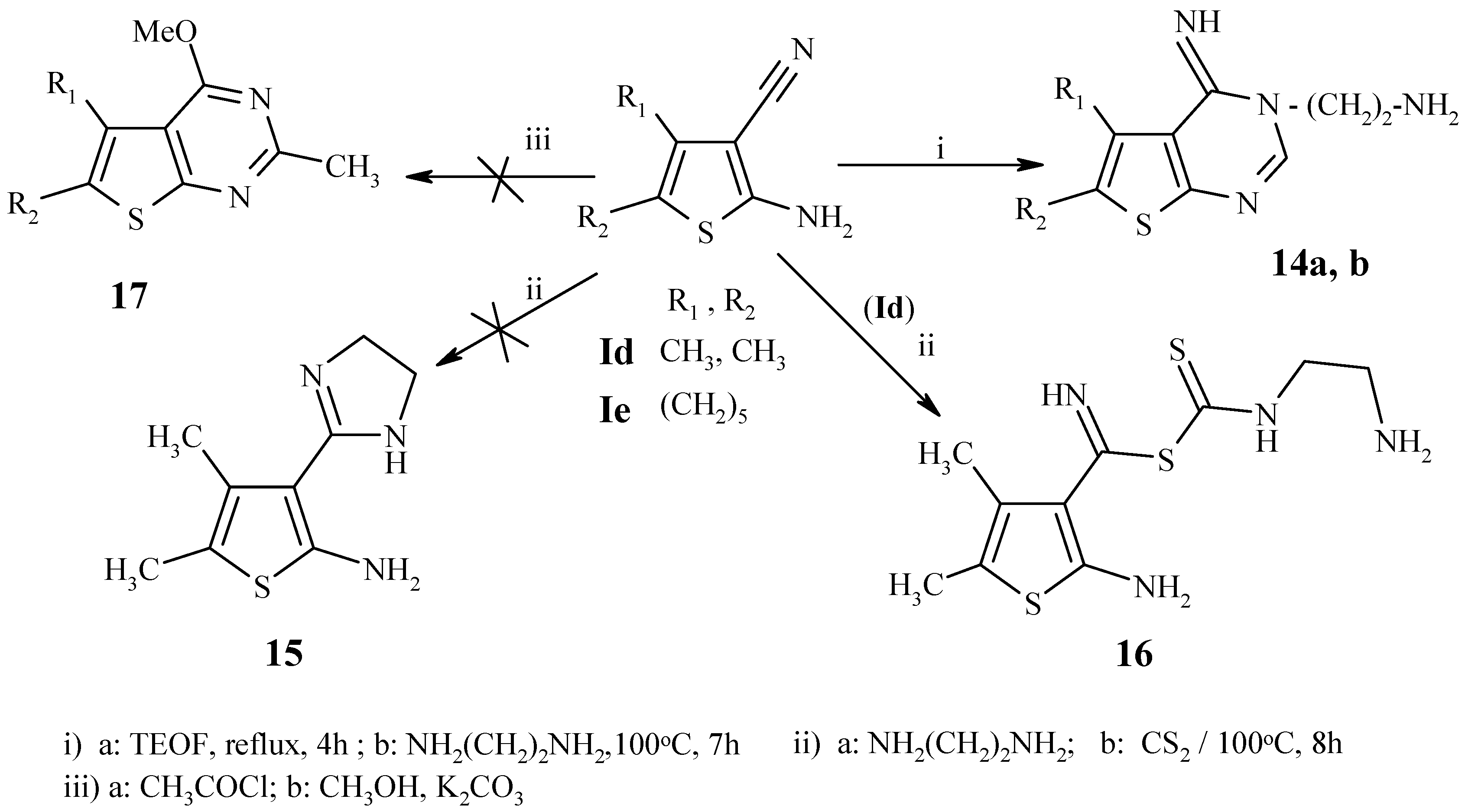
General procedure for the synthesis of 14a,b:
A mixture of 1d,e (20 mmol) and triethyl orthoformate (20 mL) was heated under reflux for 4h, and then evaporated to dryness under reduced pressure. Ethylenediamine (3 g, 50 mmol) was added dropwise with stirring and the reaction mixture was heated at 100°C for 7 h. The solid product that separated after cooling was collected by filtration, washed with ethanol, dried and recrystallized from DMF.
3-(2-Aminoethyl)-4-imino-5,6-dimethyl-3,4-dihydrothieno[2,3-d]pyrimidine (14a): Fine colorless needles, m.p. 300-302 °C; Yield 40%; IR (cm-1): 3437-3312 (NH, NH2), 1573, 1564 (2C=N); MS: m/z (%) 222 [M+] (0.22) (C10H14N4S), 205 [M-NH3] (17), 180 [M-NH3-C2H4+3H] (100).
3-(2-Aminoethyl)-4-imino-3,5,6,7,8,9-hexahydro-4H-cyclohepta[4,5]-thieno[2,3-d]pyrimidine (14b): Fine colorless needles, m.p. 218-220 °C; Yield 75%; IR (cm-1): 3371-3319 (NH, NH2), 1580, 1555 (2C=N); MS: m/z (%) 262 [M+] (0.4) (C13H18N4S), 245 [M-NH3] (22), 220 [M-NH3-C2H4+3H] (100).
Attempted preparation of 2-amino-4,5-dimethyl-3-(4,5-dihydro-1H-2-imidazolyl)thiophene (15):
Carbon disulfide (1 g, 13 mmol) was added gradually to a suspension of 1d (0.3 g, 2 mmol) and ethylenediamine (3 g, 50 mmol). The mixture was heated at 100 °C, for 8 h; the solid formed after cooling was filtered off and washed several times with DMF to give 16 as fine yellow needles, m.p. 217-219 °C; Yield 90%; IR (cm-1): 3245-3167 (NH2, NH), 1207 (C=S); MS: m/z (%) 289 [M+H] (0.2), 272 [M-NH2] (100), 254 [M-H2S-H] (37), 241 [M-H2S-CH3+2H], 221 [M-H2S-CH3-NH2-2H] (34), 162 [M-C6H8NS] (100), 137 [M-C7H9N2S+2H] (35), 102 [M-C7H10N2S2] (40).