Novel Reaction of N,N'-Bisarylmethanediamines with Formaldehyde. Synthesis of Some New 1,3,5-Triaryl-1,3,5-hexahydrotriazines
Abstract
:Introduction
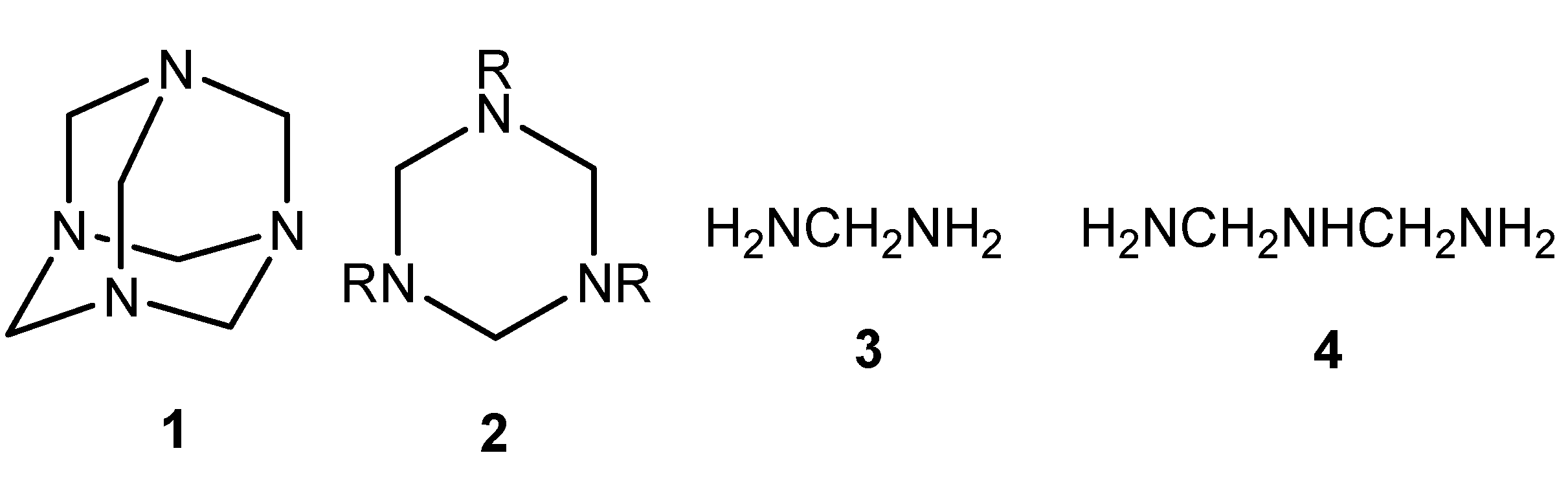
Results and Discussion
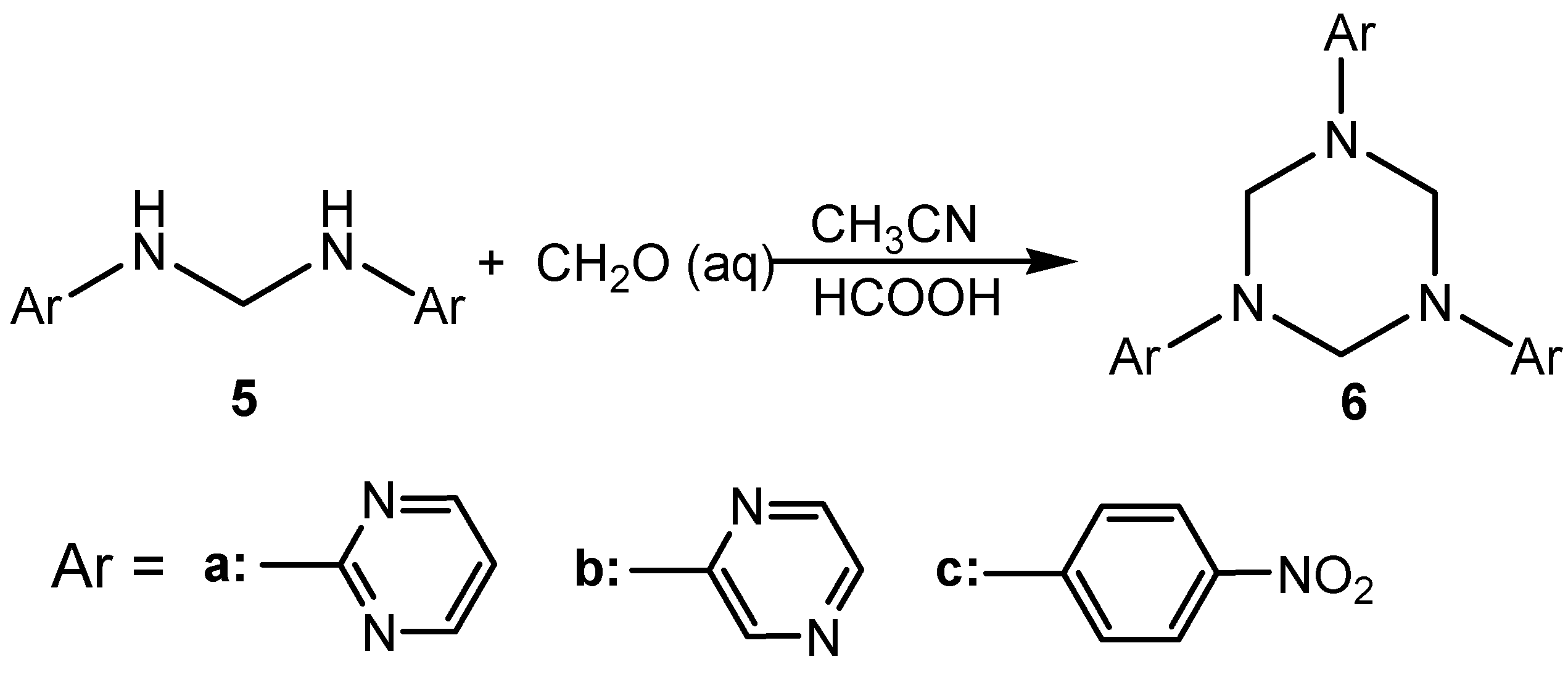
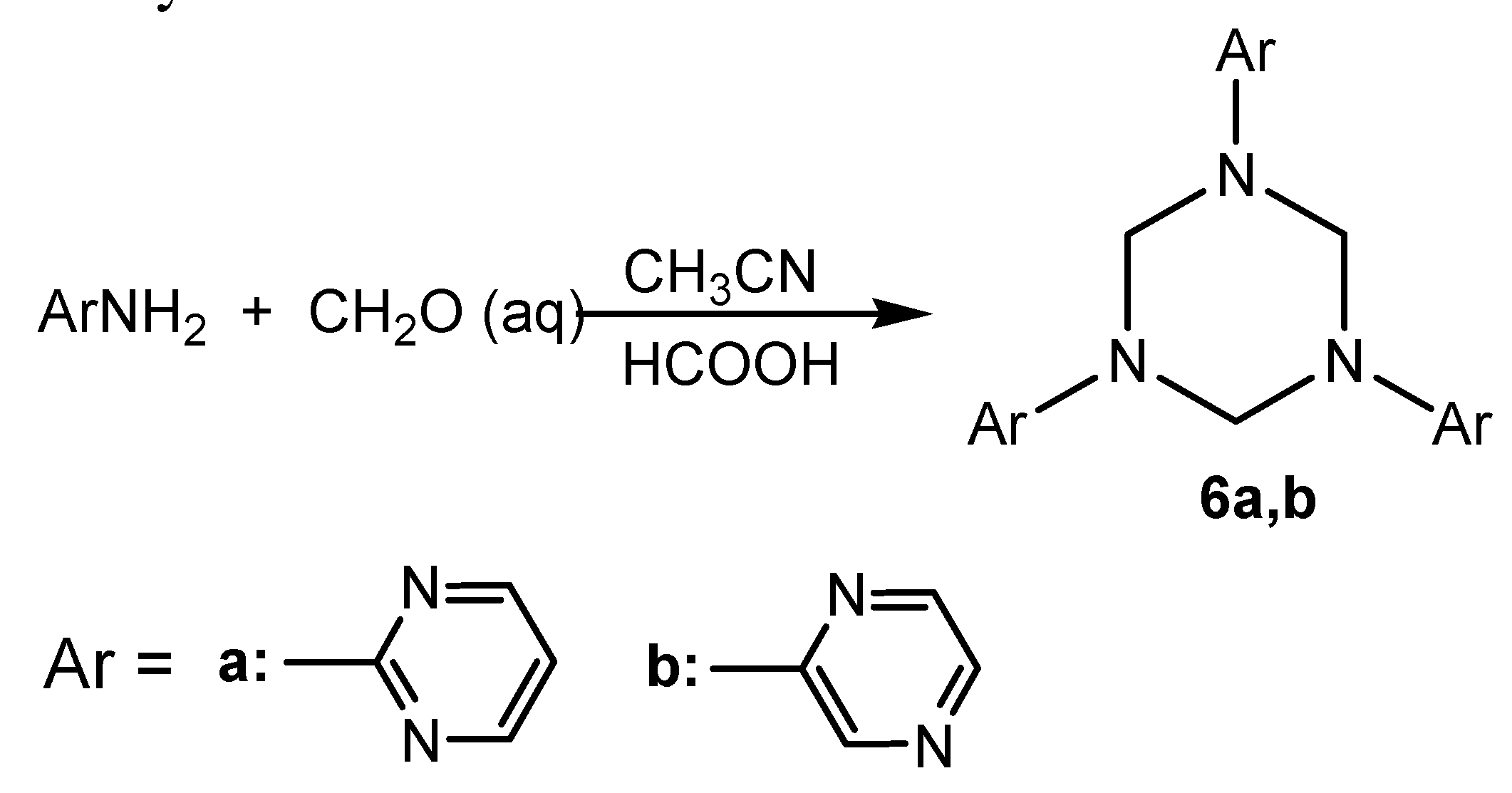
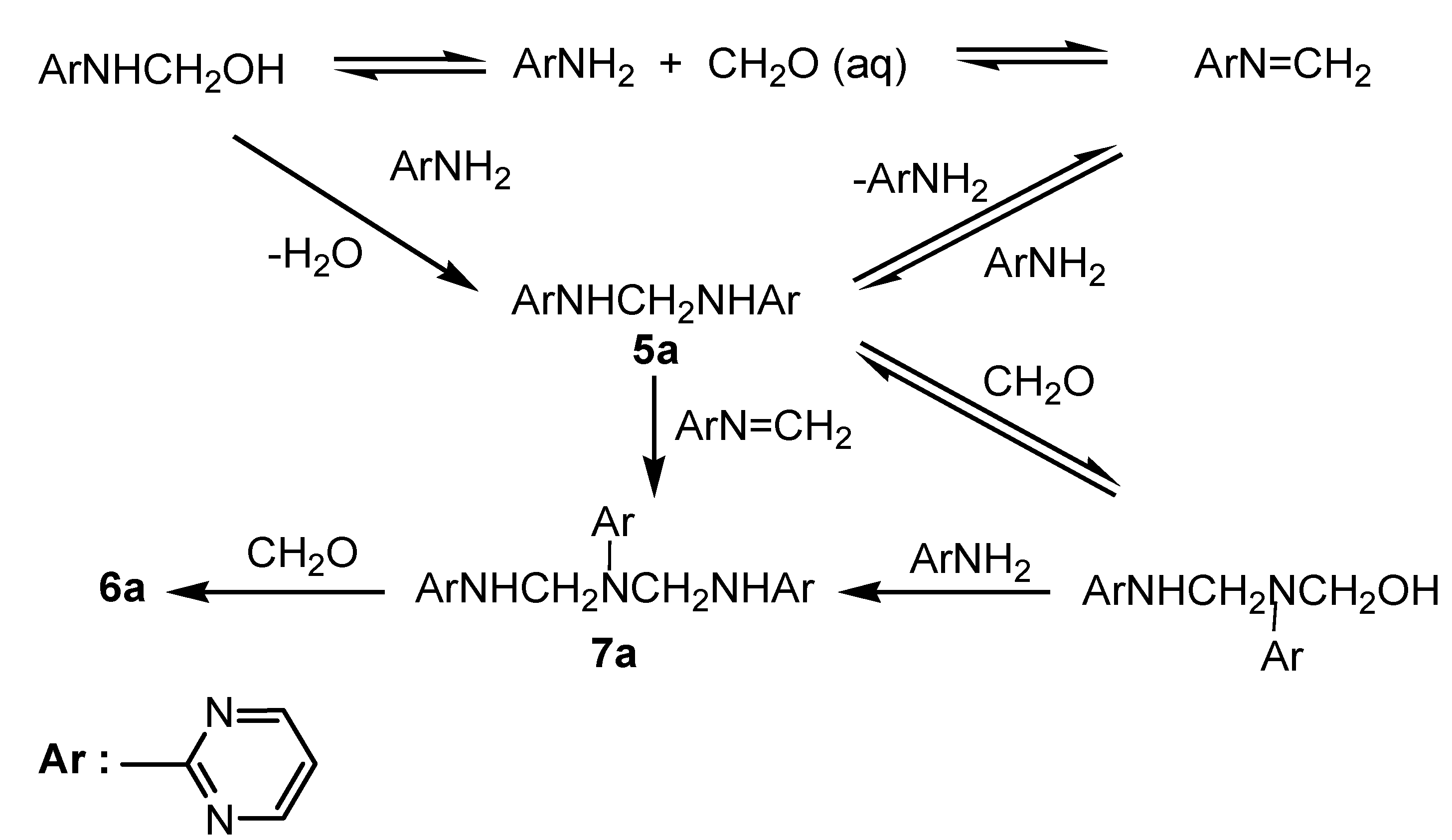
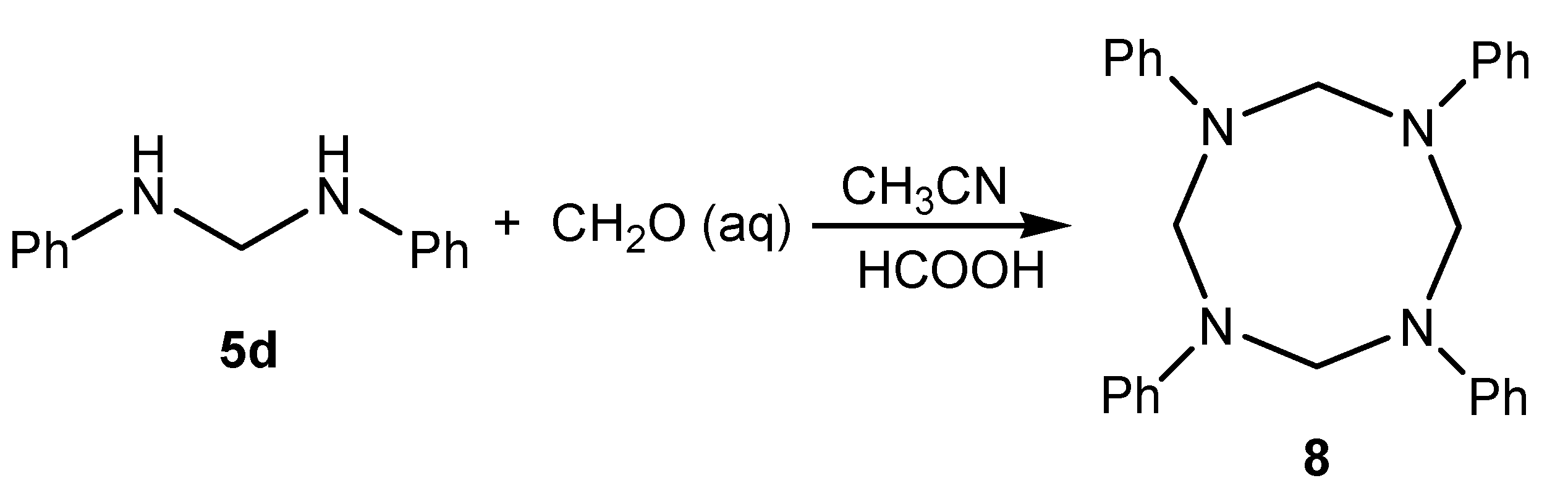
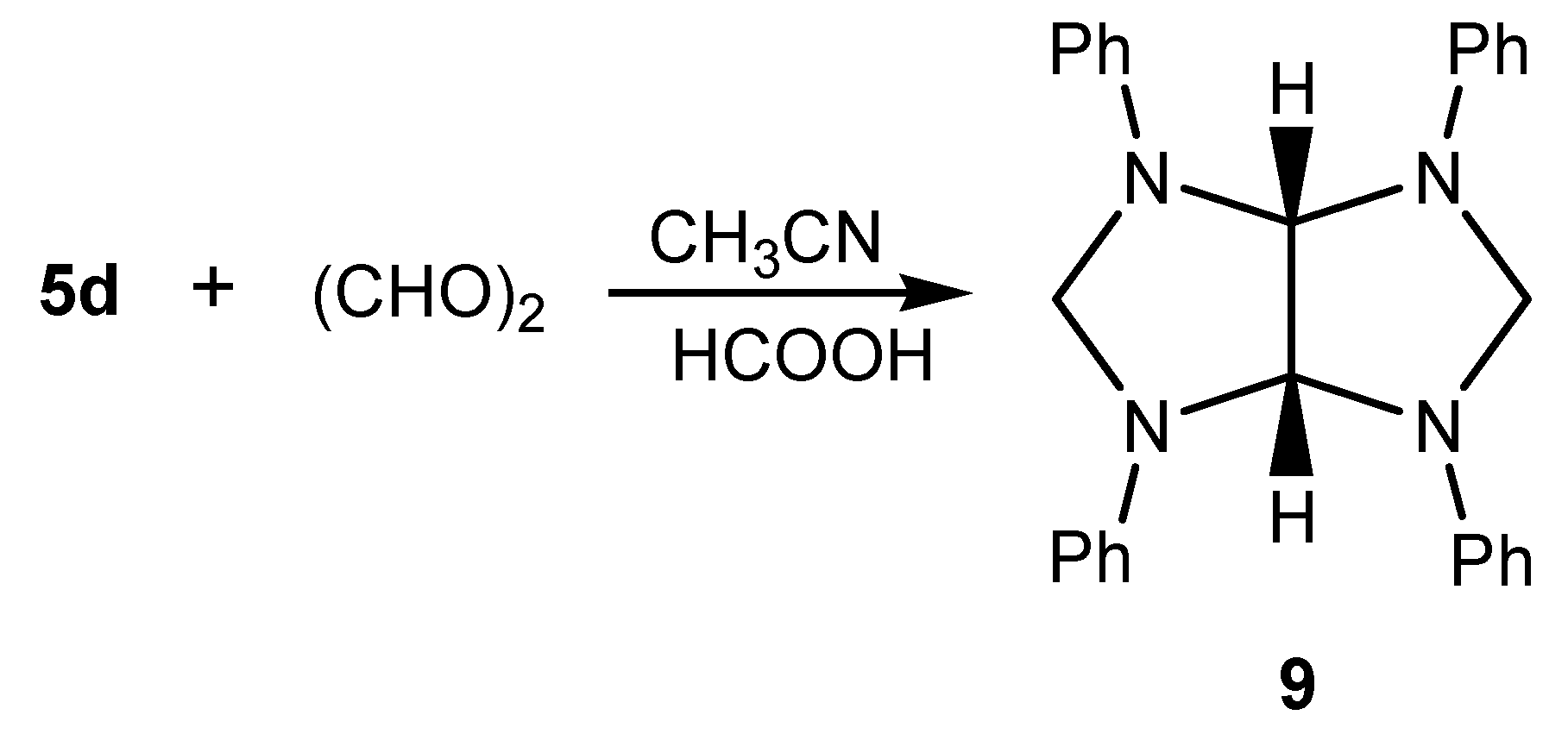
Conclusions
Experimental
General
General procedures for the synthesis of 1,3,5-triaryl-1,3,5-hexahydrotriazines 6a-c
Procedure A
Procedure B
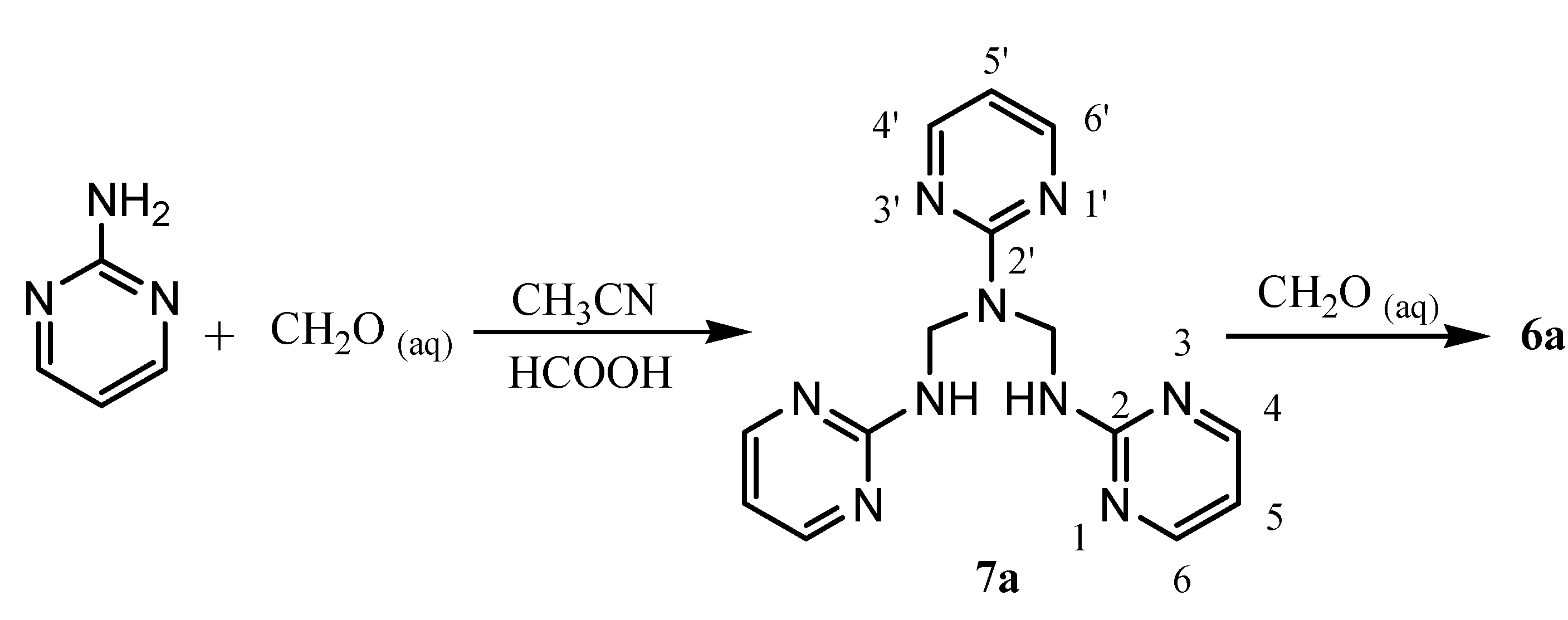
N,N',N"-Tris(2-pyrimidinyl)dimethylenetriamine (7a)
Reaction of 7a with aqueous formaldehyde
1,3,5,7-Tetraphenyltetrazocine (8)
2,4,6,8-Tetraphenyl-2,4,6,8-tetraazabicyclo[3.3.0]octane (9)
Acknowledgements
References
- Katritzky, A. R.; Suzuki, K.; He, H-Y. Convenient synthesis of unsymmetrical imidazolidines. J. Org. Chem. 2002, 67, 3109–3114. [Google Scholar] [CrossRef]
- Randaccio, L.; Zangrando, E.; Gei, M. H.; Giumanini, A. G. The cyclic tetramer of N-methyleneaniline: An X-ray diffraction structure determination. J. Prakt. Chem. 1987, 329, 187–194. [Google Scholar] [CrossRef]
- Barluenga, J.; Baylon, A. M.; Campos, P.; Asensio, G.; Gonzalez-Nunez, E.; Molina, Y. Preparation of N,O-aminals as synthetic equivalents of CH2=NR and (CH2=NHR)+ ions: Neutral-and acid-promoted transformations. J. Chem. Soc. Perkin Trans. 1 1988, 1631–1636. [Google Scholar]
- Burke, W. J.; Murdock, K. C.; Ec, G. Condensation of hydroxyaromatic compounds with formaldehyde and primary aromatic amines. J. Am. Chem. Soc. 1954, 76, 1677–1679. [Google Scholar] [CrossRef]
- Larter, M. L.; Phillips, M.; Ortega, F.; Aguirre, G.; Somanathan, R.; Walsh, P. J. Synthesis of racemic cis and trans 2,4,5-tripyridyl imidazolidines. Tetrahedron Letters 1998, 39, 4785–4788. [Google Scholar]
- Nielsen, A. T.; Atkins, R. L.; Moore, D. W.; Scott, R.; Mallory, D.; LaBerg, J. M. Structure and chemistry of the aldehyde ammonias. 1-Amino-1-alkanols, 2,4,6-trialkylhexahydro-1,3,5-triazines, and N,N-dialkylidene-1,1-diaminoalkanes. J. Org. Chem. 1973, 38, 3288–3295. [Google Scholar] [CrossRef]
- Nielsen, A. T.; Atkins, R. L.; DiPol, J.; Moore, D. W. Structure and chemistry of the aldehyde ammonias. II. Phenyl acetaldimines, styrylamines, and 2,4,6-tribenzyl-1,3,5-hexahydrotriazines. J. Org. Chem. 1974, 39, 1349–1355. [Google Scholar] [CrossRef]
- Marzona, M.; Carpignano, R. Condensation of heterocyclic amines with formaldehyde. Ann. Chim. 1966, 56, 531–539. [Google Scholar]
- Nielsen, A. T.; Moore, D. W.; Ogan, M. D.; Atkins, R. L. Structure and chemistry of the aldehyde ammonias. 3. Formaldehyde-ammonia reaction. 1,3,5-Hexahydrotriazine. J. Org. Chem. 1979, 44, 1678–1684. [Google Scholar] [CrossRef]
- Richmond, H. H.; Myers, G. S.; Wright, G. F. Reaction between formaldehyde and ammonia. J. Am. Chem. Soc. 1948, 70, 3659–3664. [Google Scholar] [CrossRef]
- Lynch, J. C.; Myers, K. F.; Brannon, J. M.; Delfino, J. J. Effects of pH and temperature on the aqueous solubility and dissolution rate of 2,4,6-trinitrotoluene (TNT), hexahydro-1,3,5-trinitro-1,3-5-triazine (RDX) and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX). J. Chem. Eng. Data 2001, 46, 1549–1555. [Google Scholar] [CrossRef]
- Marzona, M.; Carpignano, R. Condensation of heterocyclic amines with formaldehyde. Ann. Chim. 1965, 55, 1007–1013. [Google Scholar]
- Skowronska-Serafinowa, B. Some heterocyclic derivatives of methylene diamine. Rocz. Chem. 1955, 29, 932–933. [Google Scholar]
- Hunt, W. C.; Wagner, E. C. The action of acids on alkylidenebisamines. J. Org. Chem. 1951, 16, 1792–1804. [Google Scholar] [CrossRef]
- Pedersen, C. J. Thermal decomposition of crystalline tertiary butyl N-methyl-N-(p-nitrophenyl)peroxy carbamate. J. Org. Chem. 1958, 23, 255–261. [Google Scholar]
- Lerch, U.; Moffatt, J. G. Carbodiimid-sulfoxide reactions. XIII. Reactions of amines and hydrazine derivatives. J. Org. Chem. 1971, 36, 3861–3869. [Google Scholar] [CrossRef]
- Kakanejadifard, A.; Farnia, S. M. F. Synthesis and X-ray structural determination of new aniline derivatives of 2,4,6,8-tetraazabicyclo[3.3.0]octanes; anomeric effect in N-C-N moiety and implications of solvent polarity on 1H-NMR patterns. Tetrahedron 1997, 53, 2551–2556. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compounds 6a, 6b and 7a are available from authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Ghandi, M.; Salimi, F.; Olyaei, A. Novel Reaction of N,N'-Bisarylmethanediamines with Formaldehyde. Synthesis of Some New 1,3,5-Triaryl-1,3,5-hexahydrotriazines. Molecules 2006, 11, 556-563. https://doi.org/10.3390/11070556
Ghandi M, Salimi F, Olyaei A. Novel Reaction of N,N'-Bisarylmethanediamines with Formaldehyde. Synthesis of Some New 1,3,5-Triaryl-1,3,5-hexahydrotriazines. Molecules. 2006; 11(7):556-563. https://doi.org/10.3390/11070556
Chicago/Turabian StyleGhandi, Mehdi, Farshid Salimi, and Abolfazl Olyaei. 2006. "Novel Reaction of N,N'-Bisarylmethanediamines with Formaldehyde. Synthesis of Some New 1,3,5-Triaryl-1,3,5-hexahydrotriazines" Molecules 11, no. 7: 556-563. https://doi.org/10.3390/11070556



