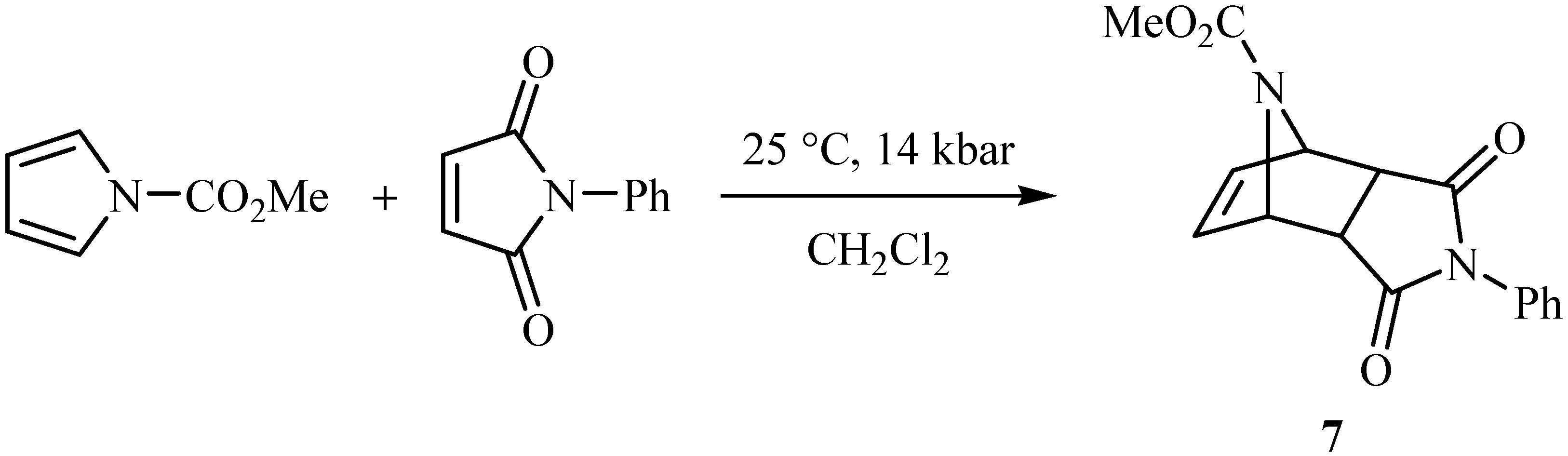Experimental
General
NMR spectra (CDCl3 solvent) were recorded on Bruker Digital FT-NMR Avance 400 and Varian Inova 500 MHz NMR spectrometers, with TMS as internal reference. In the 13C-NMR spectra quaternary, methylene and methyl carbons were identified using DEPT experiments. FTIR spectra (KBr) were recorded on a Perkin Elmer FT-IR spectrometer. GC-EIMS spectra were measured on a Varian SAT2100T/GC3900 spectrometer using ionisation by FAB. Reactions were performed under dry nitrogen. Melting points were measured on a Gallenkamp melting point apparatus. Silicagel 60 (Merck) was used for column chromatography separations. TLC was conducted on standard aluminium sheets pre-coated with a 0.2 mm layer of silica gel.
Heck Reactions – General procedure
A solution of palladium(II) acetate [Pd(OAc)2] (5.6 mg, 25 μmol) and AsPh3 (33.7 mg, 11 μmol) in anhydrous DMF or DMSO (3 mL) was stirred under nitrogen at 65 oC for 15 min. Then, compounds 3 or 7 (1.0 mmol), Et3N (488 µL, 3.5 mmol), the appropriate aryl(heteroaryl) iodide (1.5 mmol) and HCOOH (138 mg, 3.0 mmol) were added. The reaction mixture was stirred for 8-24 h. After cooling to r.t. EtOAc and brine added, the organic layer was separated, dried (MgSO4), filtered and the solvent evaporated. The residue was purified by column chromatography.
Domino-Heck Reactions – General Procedure
Pd(OAc)2 (5.6 mg, 25 μmol) and the arsine ligand (55 μmol) were dissolved in dry DMF (3 mL) and the solution was stirred at 40 oC for 15 min. Then, 3 or 7 (1.0 mmol), the aryl compound (1.5 mmol), triethylamine (488 μL, 3.50 mmol) and trimethylsilylacetylene (3.00 mmol) were added rapidly in one portion. The mixture was heated at the same temperature for 24 h. After cooling down to r.t. brine (50 mL) was added, the reaction mixture was extracted with ethyl acetate and dried over MgSO4. The solvent was evaporated and the residue purified by column chromatography.
N-Benzoyl-2-azabicyclo[2.2.1]hept-5-ene (3)
Freshly distilled cyclopentadiene (4 mL, 50 mmol) was added to a solution of NH4Cl (1.325 g, 25 mmol), 36% aqueous formaldehyde (2.6 mL, 35 mmol) and MeOH (5 mL). The reaction mixture was stirred overnight at r.t. The resulting light yellow solution was diluted with an equal volume of water and washed with diethyl ether (2x15 mL). To a mixture of this extract and 10% NaOH (5 mL) benzoyl chloride (2.10 g, 10 mmol) was added over 10 min at r.t. and the mixture was allowed to stir for 2 h. The organic layer was separated, dried over Na2SO4, filtered and the solvent removed under reduced pressure to give a yellow oil that was separated by column chromatography (1:1 ethyl acetate-n-hexane) to give 3 in 83% yield; colorless crystals; Rf: 0.57; mp 49-51 oC; IR: 3060, 1622, 1575, 1495, 1426, 1176, 710, 659 cm-1; 1H-NMR (rotamer ratio= 1:0.6) δ: 1.56-1.62 (dd, J=8.0, 6.5 Hz, 4H, H7a and H7s), [2.54-2.56 (dd, J=1.5, 1.5 Hz, minor rotamer) and 2.90-2.92 (dd, J=1.5, 1.5 Hz, major rotamer), 2H, H3n], [3.14 (bs, minor rotamer) and 3.24 (bs, major rotamer), 2H, H4], 3.49-3.55 (m, major and minor rotamers, 2H, H3x), [4.46 (bs, major rotamer) and 5.14 (bs, minor rotamer), 2H, H1], [6.18-6.20 (dd, J=2.0, 2.0 Hz, minor rotamer) and 6.20-6.22 (dd, J=2.0, 2.0 Hz, major rotamer, 2H, H6)], [6.34-6.36 (dd, J=2.0, 2.0 Hz, major rotamer) and 6.48-6.50 (dd, J=2.0, 2.0 Hz, minor rotamer), 2H, H5], 7.30-7.45 (m, 10H, aromatic protons); 13C-NMR δ : 42.8, 45.9, 49.2, 64.0, 127.3, 128.5, 128.6, 133.4, 137.3, 138.5, 171.3 (major rotamer); 44.0, 47.6, 49.8, 60.5, 127.6, 128.4, 130.3, 133.2, 135.9, 136.6, 169.5 (minor rotamer); MS: m/z 199 [M+], C13H13NO.
N-Benzoyl-5-exo-phenyl-2-azabicyclo[2.2.1]heptane (4a).
Purified by column chromatography (1:1 ethyl acetate-n-hexane) as colorless crystals in 91% yield; Rf: 0.42; mp 81-83 oC; IR: 3030, 1622, 1575, 1494, 1423, 1195, 710, 653 cm-1; 1H-NMR (rotamer ratio=1:0.5) δ: 1.51-1.70 (m, major and minor rotamers, 4H, H7a and H7s), [1.76-1.86 (m, major rotamer) and 1.92-2.20 (m, minor rotamer), 2H, H6x], [2.26-2.32 (ddt, J=2.0, 2.5 Hz, major rotamer) and 2.38-2.45 (ddt, J=2.5, 2.5 Hz, minor rotamer), 2H, H6n], [2.60 (bs, minor rotamer) and 2.74 (bs, major rotamer), 2H, H4], [2.90-2.96 (m, minor rotamer) and 3.06-3.14 (m, major rotamer), 2H, H5n], [3.24-3.28 (m, minor rotamer) and 3.32-3.36 (m, major rotamer), 2H, H3n], [3.40-3.48 (m, minor rotamer) and 3.54-3.64 (m, major rotamer), 2H, H3x], [4.08 (bs, major rotamer) and 4.74 (bs, minor rotamer), 2H, H1], [7.08-7.27 (m, major and minor rotamers), 10H, aromatic protons], [7.30-7.51 (m, major and minor rotamers), 10H, aromatic protons]; 13C-NMR δ: 35.2, 36.4, 40.6, 42.6, 53.5, 57.4, 126.6, 126.9, 127.2, 127.4, 128.6, 128.8, 130.1, 137.1, 144.9, 169.2 (major rotamer); 35.0, 35.7, 39.0, 44.1, 52.4, 56.9, 126.4, 127.0, 127.1, 127.4, 128.5, 128.8, 130.1, 135.4, 142.5, 169.6 (minor rotamer); MS: m/z 277 [M+], C19H19NO.
N-Benzoyl-5-exo-(2-thienyl)-2-azabicyclo[2.2.1]heptane (4b).
Separated by column chromatography (4:1 ethyl acetate-n-hexane) as a yellow oil, yield 57%; Rf: 0.67; IR: 3028, 1624, 1575, 1424, 1197, 798, 700 cm-1; 1H-NMR (rotamer ratio=1:0.4) δ: 1.58-1.74 (m, major and minor rotamers, 4H, H7a and H7s), 1.80-1.89 (m, major and minor rotamers, 2H, H6x), [2.30-2.36 (ddt, J=2.5, 2.5 Hz, major rotamer) and 2.42-2.48 (ddt, J=2.0, 2.0 Hz, minor rotamer), 2H, H6n], [2.58 (bs, minor rotamer) and 2.72 (bs, major rotamer), 2H, H4], 3.13-3.19 (m, major and minor rotamers, 2H, H5n), 3.23-3.34 (m, major and minor rotamers, 2H, H3n], [3.41-3.46 (dd, J=3.5, 3.0 Hz minor rotamer) and 3.59-3.62 (dd, J=3.5, 3.5 Hz, major rotamer), 2H, H3x], [4.15 (bs, major rotamer) and 4.74 (bs, minor rotamer), 2H, H1], 6.72-6.75 (m, major and minor rotamers, 2H, thienyl protons), 6.83-6.88 (m, major and minor rotamers, 2H, thienyl protons), 7.04-7.08 (m, major and minor rotamers, 2H, thienyl protons), 7.30-7.46 (m, major and minor rotamers, 10H, aromatic protons); 13C-NMR δ: 36.2, 41.3, 41.8, 44.4, 52.5, 59.8, 123.1, 123.3, 126.8, 127.1, 128.4, 130.o, 136.6, 149.3, 169.0 (major rotamer); 34.8, 40.3, 41.1, 45.7, 54.5, 56.7, 123.1, 123.3, 126.8, 127.2, 128.3, 130.1, 136.5, 149.5, 169.7 (minor rotamer); MS: m/z 283 [M+], C17H17NOS.
N-Benzoyl-5-exo-(2-naphthyl)-2-azabicyclo[2.2.1]heptane (4c).
Separated by column chromatography (1:1 ethyl acetate-n-hexane) as white crystals, yield 96%; Rf: 0.42; mp 56-58 oC; IR: 3034, 1625, 1574, 1508, 1260, 779, 701 cm-1; 1H-NMR (rotamer ratio=1:0.8) δ: 1.71-1.92 (m, major and minor rotamers, 4H, H7a and H7s), [1.93-2.01 (m, major rotamer) and 2.03-2.12 (m, minor rotamer), 2H, H6x], [2.29-2.35 (dddd, J=1.5, 2.0, 2.0, 3.0 Hz, major rotamer) and 2.57-2.64 (dddd, J=2.0, 2.5, 2.5, 3.0 Hz, minor rotamer), 2H, H6n], [2.83 (bs, minor rotamer) and 2.90 (bs, major rotamer), 2H, H4], [3.18-3.22 (d, J=9.5 Hz, minor rotamer) and 3.34-3.38 (d, J=9.0 Hz, major rotamer), 2H, H5n], [3.48-3.52 (dd, J=1.0, 1.0 Hz, minor rotamer) and 3.56-3.60 (dd, J=1.0, 1.5 Hz major rotamer) 2H, H3n], [3.65-3.72 (m, minor rotamer) and 3.76-3.82 (m, major rotamer), 2H, H3x], [4.55 (bs, major rotamer) and 5.01 (bs, minor rotamer), 2H, H1], 7.11-7.20 (d, J=7.0 Hz, major rotamer, 1H, aromatic proton), 7.28-7.47 (m, major and minor rotamers, 13H, aromatic protons), 7.48-7.62 (m, major and minor rotamers, 4H aromatic protons), 7.66-7.70 (d, J=9.5 Hz, minor rotamer, 1H, aromatic proton), 7.71-7.74 (d, J=8.0 Hz, major rotamer, 1H, aromatic proton), 7.80-7.83 (d, J=8.5 Hz, minor rotamer, 1H, aromatic proton), 7.84-7.88 (d, J=8.0 Hz, major rotamer, 1H, aromatic proton), 7.98-8.02 (d, J=7.5 Hz, minor rotamer, 1H, aromatic proton), 8.04-8.08 (d, J=9.0 Hz, major rotamer, 1H, aromatic proton); 13C-NMR δ: 35.1, 37.0, 39.4, 41.4, 53.5, 60.5, 121.4, 124.0, 125.5, 125.9, 126.3, 127.3, 127.5, 128.7, 129.2, 130.2, 131.8, 134.2, 137.0, 140.6, 169.2 (major rotamer); 34.7, 37.0, 39.7, 41.3, 52.9, 60.9, 122.1, 123.7, 125.3, 125.9, 126.3, 127.2, 127.4, 128.6, 129.0, 130.2, 131.7, 134.4, 136.9, 141.0, 169.0 (minor rotamer); MS: m/z 327 [M+], C23H21NO.
N-Benzoyl-5-exo-(2-chloro-5-pyridinyl)-2-azabicyclo[2.2.1]heptane (4d).
Separated by column chromatography (1:1 ethyl acetate-n-hexane) as a colorless oil, yield 54%; Rf: 0.45; IR: 3065, 1627, 1574, 1508, 1260, 780, 714 cm-1; 1H-NMR (rotamer ratio=1:0.6) δ: 1.52-1.70 (m, major and minor rotamers, 4H, H7a and H7s), [1.78-2.20 (m, major and minor rotamers), 2H, H6x], [2.28-2.32 (dddd, J=2.0, 2.0, 2.5, 2.5 Hz, major rotamer) and 2.38-2.44 (dddd, J=2.0, 2.0, 2.5, 2.5 Hz, minor rotamer), 2H, H6n], [2.62 (bs, minor rotamer) and 2.74 (bs, major rotamer), 2H, H4], [2.90-3.00 (m, minor rotamer) and 3.06-3.15 (m, major rotamer), 2H, H5n], [3.22-3.28 (m, minor rotamer) and 3.33-3.38 (m, major rotamer) 2H, H3n], [3.43-3.48 (m, minor rotamer) and 3.52-3.62 (m, major rotamer), 2H, H3x], [4.14 (bs, major rotamer) and 4.78 (bs, minor rotamer), 2H, H1], 7.26-7.28 (d, J=8.0 Hz, major and minor rotamers, 2H, aromatic protons), 7.30-7.47 (m, major and minor rotamers, 10H, aromatic protons), 7.47-7.49 (dd, J= 8.0, 3.0 Hz, 2H, major and minor rotamers, aromatic protons), 8.22-8.24 (d, J=3.0 Hz, 2H, major and minor rotamers, aromatic protons); 13C-NMR δ: 35.1, 36.4, 40.5, 42.7, 53.4, 57.5, 126.6, 126.9, 127.1, 128.5, 128.7, 130.0, 136.7, 139.2, 148.4, 169.1 (major rotamer), 35.0, 36.2, 40.8, 42.9, 54.0, 57.9, 127.0, 127.2, 128.9, 129.0, 130.5, 137.0, 139.8, 148.7, 170.2 (minor rotamer); MS: m/z 312 [M+], C18H17ClN2O.
N-Benzoyl-6-exo-(2-thienyl)-2-azabicyclo[2.2.1]heptane (5b).
Separated by column chromatography (4:1 ethyl acetate-n-hexane) as yellow crystals, yield 40%; Rf: 0.60; mp 98-100 oC, IR: 3025, 1613, 1571, 1430, 1074, 789, 716 cm-1; 1H-NMR (rotamer ratio= 1:0.3) δ: 1.52-1.66 (m, major and minor rotamers, 4H, H7a and H7s), 1.92-1.98 (m, major and minor rotamers, 2H, H5x), 2.08-2.14 (m, major and minor rotamers, 2H, H5n), [2.60 (bs, minor rotamer) and 2.72 (bs, major rotamer), 2H, H4], [3.08-3.11 (dd, J=1.0, 1.0 Hz, minor rotamer) and 3.20-3.23 (dd, J=1.5, 1.5 Hz major rotamer), 2H, H3n], [3.40-3.43 (tt, minor rotamer) and 3.49-3.52 (m, major rotamer), 2H, H3x], 3.54-3.60 (m, major and minor rotamers, 2H, H6n), [4.04 (bs, major rotamer) and 4.70 (bs, minor rotamer), 2H, H1], 6.82-6.90 (dd, J=1.0, 1.0 Hz, major and minor rotamers, 2H, thienyl protons), 7.04-7.10 (dd, J=1.0, 1.0 Hz, major and minor rotamers, 4H, thienyl protons), 7.28-7.52 (m, major and minor rotamers, 10H, aromatic protons); 13C-NMR δ: 35.7, 37.1, 39.2, 45.0, 52.1, 65.8, 123.4, 123.6, 127.0, 127.1, 128.4, 130.2, 136.2, 146.6, 168.9 (major rotamer), 36.2, 37.5, 39.5, 43.1, 56.9, 62.2, 123.3, 123.8, 126.9, 127.3, 128.3, 130.2, 136.6, 147.6, 169.5 (minor rotamer); MS: m/z 283 [M+], C17H17NOS.
N-Benzoyl-6-exo-(2-chloro-5-pyridinyl)-2-azabicyclo[2.2.1]heptane (5d).
Separated by column chromatography (1:1 ethyl acetate-n-hexane) as a colorless oil, yield 40%; Rf: 0.39; IR: 3065, 1625, 1575, 1510, 1260, 781, 712 cm-1; 1H-NMR (rotamer ratio=1:0.55) δ: 1.52-1.68 (m, major and minor rotamers, 4H, H7a and H7s), [1.92-2.00 (m, major rotamer and minor rotamers), 2H, H5x], [2.02-2.12 (m, major rotamer and minor rotamers), 2H, H5n], [2.60 (bs, minor rotamer) and 2.74 (bs, major rotamer), 2H, H4], [3.08-3.13 (dd, J= 1.0, 1.0 Hz, minor rotamer) and 3.19-3.24 (dd, J=1.5, 1.5 Hz, major rotamer), 2H, H3n], [3.40-3.45 (tt, minor rotamer) and 3.48-3.52 (tt, major rotamer) 2H, H3x], 3.54-3.58 (m, major rotamer and minor rotamers), 2H, H6n], [4.16 (bs, major rotamer) and 4.72 (bs, minor rotamer), 2H, H1], 7.20-7.40 (m, major and minor rotamers, 12H, aromatic protons), 7.41-7.50 (dd, J=8.5, 2.5 Hz, major and minor rotamers, 2H, aromatic protons), 8.16-8.18 (d, J=2.5 Hz, major and minor rotamers, 2H, aromatic protons); 13C-NMR δ: 35.2, 36.5, 37.2, 45.1, 52.0, 62.0, 126.8, 127.8, 128.1, 128.6, 130.1, 136.9, 145.3, 149.4, 169.7 (major rotamer), 35.4, 36.8, 37.8, 45.7, 52.3, 61.8, 127.0, 128.0, 128.2, 128.9, 131.0, 137.4, 145.6, 149.8, 170.0 (minor rotamer); MS: m/z 312 [M+], C18H17ClN2O.
N-Benzoyl-5-exo-(trimethylsilylethynyl)-6-exo-phenyl-2-azabicyclo[2.2.1]heptane (6e).
Separated by column chromatography (2:3 ethyl acetate-n-hexane) as yellow crystals, yield 60%; Rf: 0.38; mp 113-115 oC; IR: 3025, 2177, 1629, 1574, 1427, 1085, 840, 696 cm-1; 1H-NMR (rotamer ratio=1:0.4) δ: -0.154 (s, major rotamer, 9H, Si(CH3)3), -0.145 (s, minor rotamer, 9H, Si(CH3)3), 1.81-1.89 (m, major and minor rotamers, 4H, H7a and H7s), [2.19-2.22 (d, J=10.4 Hz, major rotamer) and 2.29-2.31 (d, J=10.8 Hz, minor rotamer), 2H, H6n], [2.78 (bs, minor rotamer) and 2.89 (bs, major rotamer), 2H, H4], [3.11-3.13 (m, minor rotamer) and 3.24-3.31 (m, major rotamer), 2H, H5n], 3.46-3.50 (dd, J=4.0, 3.6 Hz, minor rotamer, 1H, H3n), [3.69-3.72 (dd, J=4.0, 4.0 Hz, major rotamer) and 3.51-3.53 (d, J=9.6, minor rotamer), 2H, H3x], 3.55-3.57 (d, J=9.2 Hz, major rotamer, 1H, H3n), [4.40 (bs, major rotamer) and 5.05 (bs, minor rotamer), 2H, H1], 7.06-7.28 (m, major and minor rotamers, 10H, aromatic protons), 7.29-7.54 (m, major and minor rotamers, 10H, aromatic protons); 13C-NMR δ: -0.42, 36.4, 40.9, 43.6, 50.9, 53.5, 62.6, 89.6, 106.0, 126.4, 127.1, 127.9, 128.0, 128.4, 130.0, 136.0, 139.0, 168.3 (major rotamer), -0.51, 34.9, 40.6, 44.9, 51.9, 54.5, 58.8, 89.6, 106.3, 126.2, 127.2, 127.7, 128.3, 128.4, 130.1, 136.1, 139.8, 169.2 (minor rotamer); MS: m/z 373[M+], C24H27SiNO.
N-Benzoyl-6-exo-(trimethylsilylethynyl)-exo-5-phenyl-2-azabicyclo[2.2.1]heptane (6f).
Separated by column chromatography (2:3 ethyl acetate-n-hexane) as yellow crystals, yield 35%; Rf: 0.43; mp 128-130 oC; IR: 3026, 2171, 1626, 1573, 1418, 1072, 835, 695 cm-1; 1H-NMR (rotamer ratio=1:0.4) δ: -0.199 (s, major rotamer, 9H, Si(CH3)3), -0.147 (s, minor rotamer, 9H, Si(CH3)3), [1.84-1.86 (m, major rotamer) and 1.90-1.93 (m, minor rotamer), 4H, H7a and H7s), [2.26-2.29 (d, J=10.0 Hz, major rotamer) and 2.37-2.39 (d, J=10.4 Hz, minor rotamer), 2H, H6n], [2.83 (bs, minor rotamer) and 2.98 (bs, major rotamer), 2H, H4], 3.13-3.16 (m, major and minor rotamers, 2H, H5n), 3.37-3.41 (m, major and minor rotamers, 2H, H3n), [3.56-3.59 (dd, J=3.6, 3.2 Hz, minor rotamer) and 3.64-3.68 (dd, J=3.6, 3.2 Hz, major rotamer), 2H, H3x], [4.24 (bs, major rotamer) and 4.78 (bs, minor rotamer), 2H, H1], 7.15-7.25 (m, major and minor rotamers, 5H, aromatic protons), 7.26-7.31 (m, major and minor rotamers, 4H, aromatics), 7.40-7.48 (m, major and minor rotamers, 5H, aromatic protons), 7.52-7.56 (m, major and minor rotamer, 6H, aromatic protons); 13C-NMR δ: -0.51, 36.9, 40.2, 44.7, 50.2, 54.0, 64.4, 91.2, 103.5, 126.3, 127.1, 127.9, 128.0, 128.5, 130.1, 136.1, 140.9, 169.3 (major rotamer), -0.43, 34.8, 41.8, 43.0, 49.6, 57.8, 61.1, 90.6, 104.1, 126.2, 127.2, 127.8, 128.0, 128.3, 130.2, 136.1, 141.1, 169.8 (minor rotamer); MS: m/z 373 [M+], C24H27SiNO.
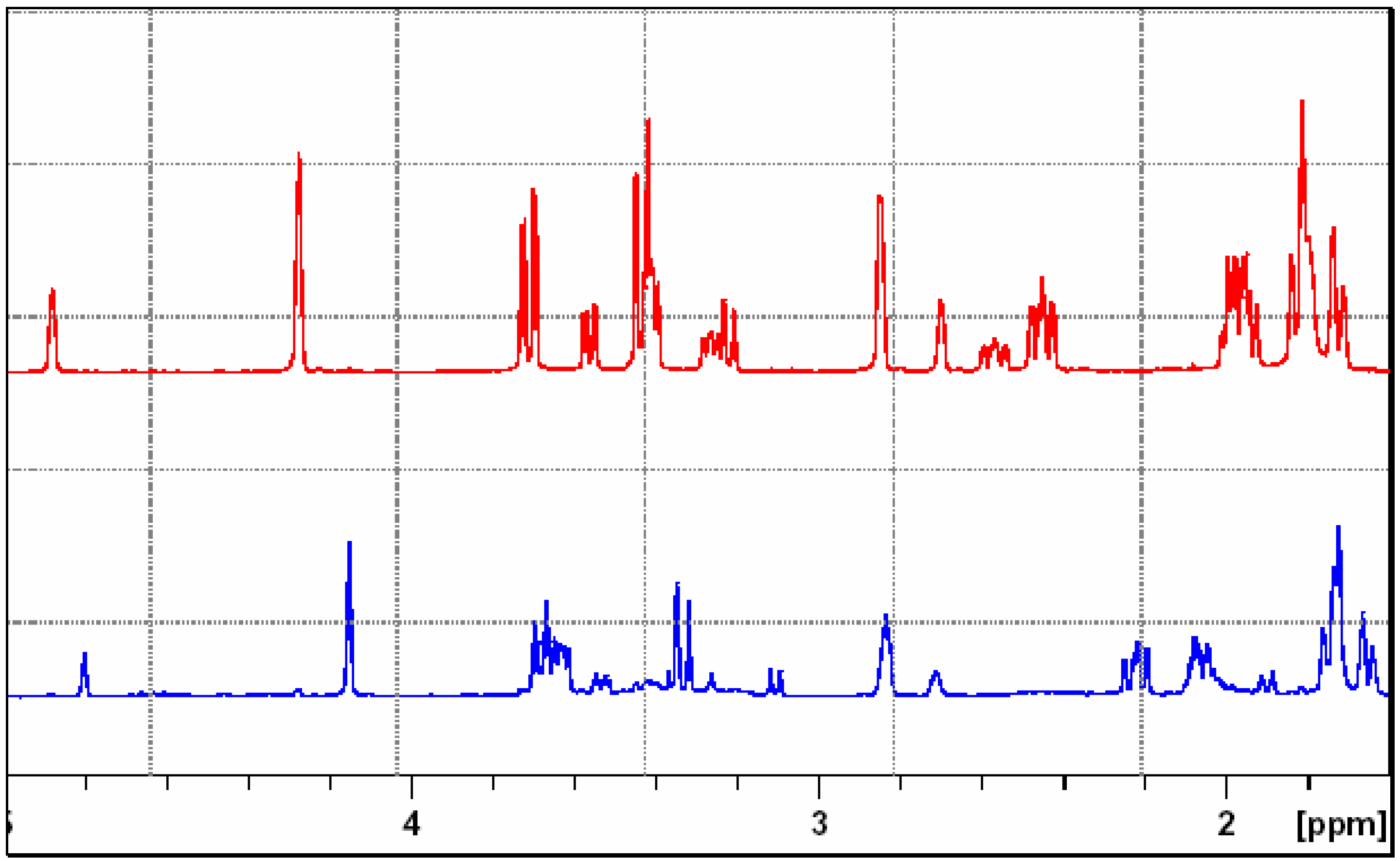
endo-Methyl 3,5-dioxo-4-phenyl-4,10-diazatricyclo[5.2.1.02,6]dec-8-ene-10-carboxylate (7).
IR: 3065, 1774, 1713, 1697 cm-1; 1H-NMR δ: 2.96 (s, 2H, H2 and H6), 3.62 (s, 3H, -OCH3), 5.21 (s, 2H, H1 and H7), 6.58 (s, 2H, H8 and H9), 7.24-7.48 (m, 5H, aromatic protons); 13C-NMR δ: 53.1, 63.5, 126.3, 128.8, 129.1, 131.5, 156.0, 174.5,175.1; MS: m/z 298 [M+], C16H14N2O4.
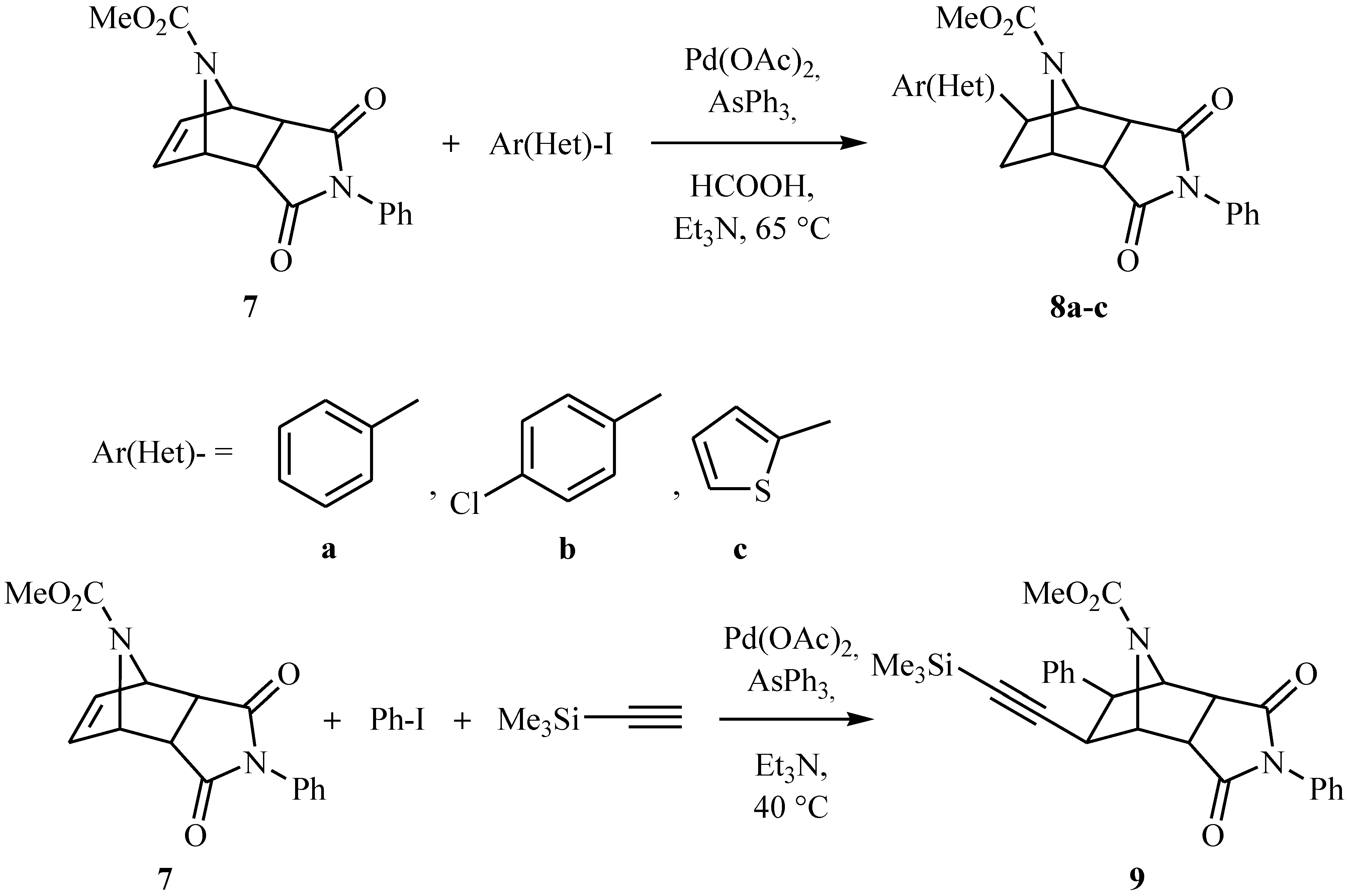
endo-Methyl 3,5-dioxo-4,8exo-diphenyl-4,10-diazatricyclo[5.2.1.02,6]decane-10-carboxylate (8a)
Separated by column chromatography (2:1 ethyl acetate-n-hexane) as colorless crystals, yield 55%; Rf: 0.49; mp 220-222 oC; IR: 3061, 1777, 1713, 1698 cm-1; 1H-NMR δ: 2.12-2.23 (m, 2H, H9x and H9n), 3.09-3.26 (m, 2H, H2 and H6), 3.46 (s, 3H, -OCH3), 3.57 (s, 1H, H8n), 4.75 (bs, 1H, H1), 5.06 (bs, 1H, H7), 7.21-7.33 (m, 5H, aromatic protons); 7.36-7.48 (m, 5H, aromatic protons); 13C-NMR δ: 37.9, 47.7, 49.3, 50.7, 52.9, 60.6, 65.3, 126.3, 127.2, 128.8, 129.3, 131.9, 142.9, 155.3, 175.2, 175.4; MS: m/z 375 [M+], C22H20N2O4.
endo-Methyl 8-exo-(4-chlorophenyl)-3,5-dioxo-4-phenyl-4,10-diazatricyclo[5.2.1.02,6]decane-10-carboxylate (8b).
Separated by column chromatography (1:1 ethyl acetate-n-hexane) as colorless crystals, yield 72%; Rf: 0.40; mp 182-84 oC; IR: 3058, 1776, 1716, 1695 cm-1; 1H-NMR (δ: 2.07-2.21 (m, 2H, H9x and H9n), 3.07-3.24 (m, 2H, H2 and H6), 3.47 (s, 3H, -OCH3), 3.57 (s, 1H, H8n), 4.70 (bs, 1H, H1), 5.06 (bs, 1H, H7), 7.23-7.29 (m, 5H, aromatic protons), 7.36-7.41 (m, 2H, aromatic protons), 7.46-7.48 (m, 2H, aromatic protons); 13C-NMR δ: 37.8, 46.8, 49.0, 50.3, 52.9, 59.2, 65.0, 126.4, 128.3, 128.6, 128.8, 129.1, 131.4, 132.9, 141.2, 155.2, 174.9, 175.0; MS: m/z 410 [M+], C22H19ClN2O4.
endo-Methyl 3,5-dioxo-4-phenyl-8-exo-(2-thienyl)-4,10-diazatricyclo[5.2.1.02,6]decane-10-carboxyl-ate (8c).
Separated by column chromatography (1:1 ethyl acetate-n-hexane) as colorless crystals, yield 58%; Rf: 0.43; mp 224-226 oC; IR: 3112, 3064, 1778, 1713, 1691 cm-1; 1H-NMR δ: 2.22-2.24 (m, 2H, H9x and H9n), 3.10-3.12 (dd, J=7.5, 4.0 Hz, 1H, H6), 3.21-3.24 (d, J=9.0 Hz, 1H, H2), 3.45 (s, 3H, -OCH3), 3.57 (s, 1H, H8n), 4.75 (bs, 1H, H1), 5.04 (bs, 1H, H7), 6.92-6.94 (dd, J=4.5, 4.5 Hz, 2H, thienyl protons), 7.24-7.26 (m, 1H, thienyl proton), 7.37-7.49 (m, 5H, aromatic protons); 13C-NMR δ: 38.7, 48.7, 49.6, 52.7, 58.9, 65.8, 124.0, 124.4, 126.0, 126.4, 126.6, 128.8, 129.1, 131.4, 146.2, 155.4, 174.9, 175.0; MS: m/z 382 [M+], C20H18N2O4S.
endo-Methyl 3,5-dioxo-9-exo-(trimethylsilylethynyl)-4,8-exo-diphenyl-4,10-diazatricyclo-[5.2.1.02,6]-decane-10-carboxylate (9).
Separated by column chromatography (1:1 ethyl acetate-n-hexane) as yellow crystals, yield 42%; Rf: 0.52; mp 196-198 oC; IR: 3033, 1781, 1717, 1693 cm-1; 1H-NMR δ: 0.0 (s, 9H, Si(CH3)3), 3.31-3.48 (m, 4H, H9n, H8n, H2 and H6), 3.78 (s, 3H, -OCH3), 5.08 (bs, 1H, H1), 5.31 (bs, 1H, H7), 7.36-7.45 (m, 5H, aromatic protons), 7.47-7.64 (m, 5H, aromatic protons); 13C-NMR δ: 0.52, 42.9, 49.0, 49.9, 51.6, 53.0, 63.1, 64.5, 91.6, 102,7, 125.9, 126.0, 127.0, 128.0, 128.3, 128.8, 129.2, 134.2, 139.0, 154.4, 174.3, 174.5; MS: m/z 472 [M+], C27H28SiN2O4.