One-Pot Synthesis of 5-Arylidene-2-Imino-4-Thiazolidinones under Microwave Irradiation
Abstract
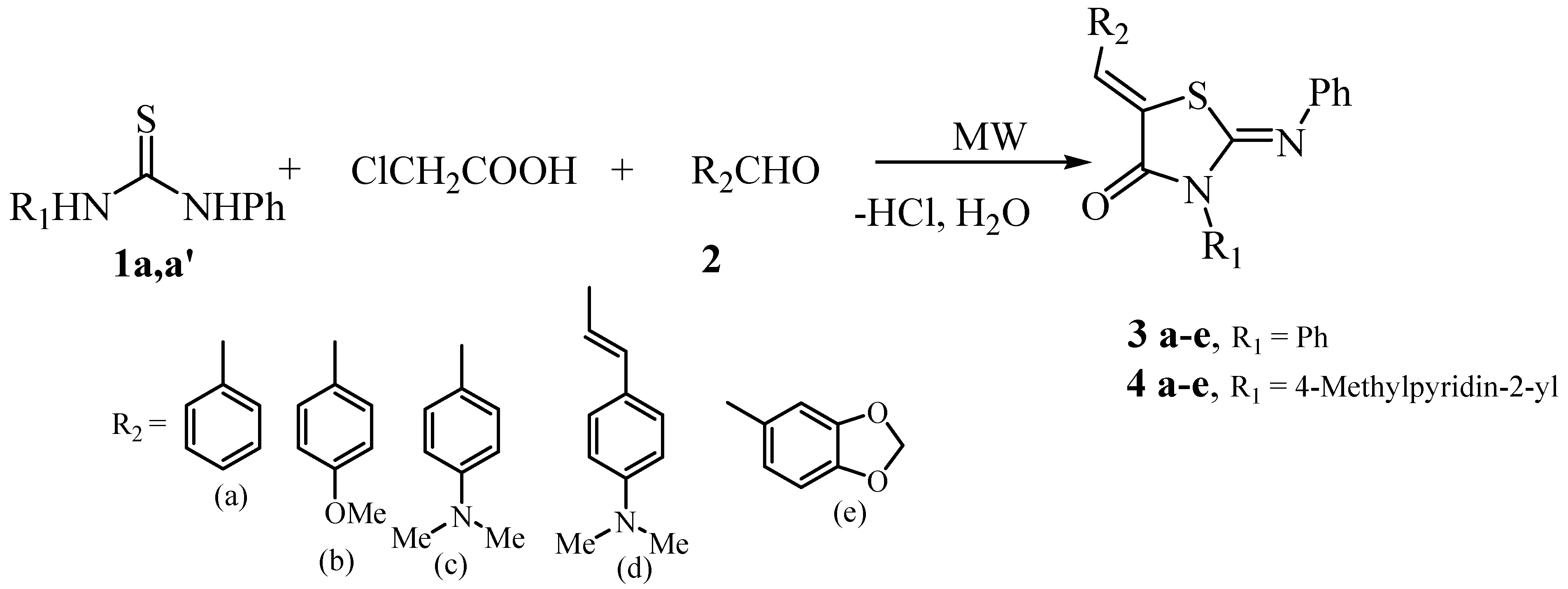
:Introduction
| Compounds 3 | R1 | Time (min) | Yields(%)(a) |
|---|---|---|---|
| 3a | Ph | 20 | 89 |
| 3b | Ph | 15 | 64 |
| 3c | Ph | 20 | 73 |
| 3d | Ph | 20 | 71 |
| 3e, 3e’ | Ph | 20 | 75(b) |
| 4a | 4-methylpyridin-2-yl | 20 | 79 |
| 4b | 4-methylpyridin-2-yl | 20 | 68 |
| 4c | 4-methylpyridin-2-yl | 10 | 77 |
| 4d | 4-methylpyridin-2-yl | 10 | 74 |
| 4e | 4-methylpyridin-2-yl | 15 | 61 |
Conclusions
Experimental
General
Preparation of Thioureas 1a,1a’
General procedure for the preparation of 5-arylidene-2-imino-4-thiazolidinones 3a-e, 4a-e.
Acknowledgements
References
- Doran, W. J.; Shonle, H. A. Dialkyl thiazolidinones. J. Org. Chem. 1938, 3, 193–197. [Google Scholar] [CrossRef]
- Troutman, H. D.; Long, L. M. The synthesis of 2,3-disubstituted-4-thiazolidinones. J. Am. Chem. Soc. 1948, 70, 3436–3439. [Google Scholar] [CrossRef]
- Vicini, P.; Geronikaki, A.; Anastasia, K.; Incerti, M.; Zani, F. Synthesis and antimicrobial activity of novel 2-thiazolylimino-5-arylidene-thiazolidinones. Bioorg. Med. Chem. 2006, 14, 3859–3864. [Google Scholar] [CrossRef]
- El-Gendy, Z.; Abdel-Rahman, R. M.; Fawzy, M. M.; Mahmoud, M. B. Biologically active thiazolidinone. Part II. Synthesis and fungitoxicities of isolated and fused thiazolidinones derived from thiosemicarbazones. J. Indian Chem. Soc. 1990, 67, 927–929. [Google Scholar]
- Zervosen, A.; Lu, W. P.; Chen, Z.; White, R. E .; Demuth, T.P., Jr.; Frère, J.M. Interaction between Penicillin- Binding Proteins (PBPs) and two novel classes of PBP inhibitors, aryl-alkylidene rhodanines and arylalkylidene iminothiazolidin-4-ones. Antimicrob. Agents Chemother. 2004, 48, 961–969. [Google Scholar] [CrossRef]
- Diurno, M. V.; Mazzoni, O.; Piscopo, E.; Calignano, A.; Giordano, F.; Bolognese, A. Synthesis and antihistaminic activity of some thiazolidin-4-ones. J. Med. Chem. 1992, 35, 2910–2912. [Google Scholar] [CrossRef]
- Bhargava, P. N.; Prakash, S.; Lakhan, R. Synthesis of 2- (4´,5´-disubstituted thiazol-2´-ylimino)-3-(methylphenyl)-5-methyl (or H)-thiazolidin-4-ones and their fungicidal activity. Indian J. Chem. 1981, 20B, 927–929. [Google Scholar]
- Liu, H-L.; Li, Z.; Anthonsen, T. Synthesis and Fungicidal Activity of 2-Imino-3-(4-aryl-thiazol-2-yl)-thiazolidin-4-ones and their 5-Arylidene Derivatives. Molecules 2000, 5, 1055–1061. [Google Scholar] [CrossRef]
- Kandeel, K. A. Synthesis and structures of some new thiazolidin-4-ones and thiazolin-4-one of anticipated biological activity. Arkivoc 2006, 1–6. [Google Scholar] D’hooge, M.; De Kimpe, N. Synthetic approaches towards 2-iminothiazolidines: an overview. Tetrahedron 2006, 62, 513–535. [Google Scholar] Laurent, D. R. St.; DedongWu, Q.G.; Serrano-Wu, H. Regioselective synthesis of 3-(hetero-aryl)-iminothiazolidin-4-ones. Tetrahedron Lett. 2004, 45, 1907–1910. [Google Scholar]
- Ottana, R.; Maccari, R.; Barreca, M.L.; Bruno, G.; Rotondo, A.; Rossi, A.; Chiricosta, G.; Di- Paola, R.; Sautebin, L.; Cuzzocrea, S.; Vigorita, M.G. 5-Arylidene-2-imino-4-thiazolidinones: Design and synthesis of novel anti-inflammatory agents. Bioorg. Med. Chem. 2005, 13, 4243–4252. [Google Scholar] [CrossRef]
- Kasmi, S.; Hamelin, J.; Benhaoua, H. Microwave-assisted solvent free synthesis of imino- thiazolines. Tetrahedron Lett. 1998, 39, 8093–8096. [Google Scholar] [CrossRef]
- Bruno, G.; Costantino, L.; Curinga, C.; Maccari, R.; Monfore, F.; Nicolo, F.; Ottana, R.; Vigorita, M.G. Bioorg. Med. Chem. 2002, 10, 1077–1084.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Kasmi-Mir, S.; Djafri, A.; Paquin, L.; Hamelin, J.; Rahmouni, M. One-Pot Synthesis of 5-Arylidene-2-Imino-4-Thiazolidinones under Microwave Irradiation. Molecules 2006, 11, 597-602. https://doi.org/10.3390/11080597
Kasmi-Mir S, Djafri A, Paquin L, Hamelin J, Rahmouni M. One-Pot Synthesis of 5-Arylidene-2-Imino-4-Thiazolidinones under Microwave Irradiation. Molecules. 2006; 11(8):597-602. https://doi.org/10.3390/11080597
Chicago/Turabian StyleKasmi-Mir, Souad, Ayada Djafri, Ludovic Paquin, Jack Hamelin, and Mustapha Rahmouni. 2006. "One-Pot Synthesis of 5-Arylidene-2-Imino-4-Thiazolidinones under Microwave Irradiation" Molecules 11, no. 8: 597-602. https://doi.org/10.3390/11080597




