A New Poly-substituted Benzaldehyde from the Leaves of Lysimachia fordiana Oliv.
Abstract
:Introduction
Results and Discussion

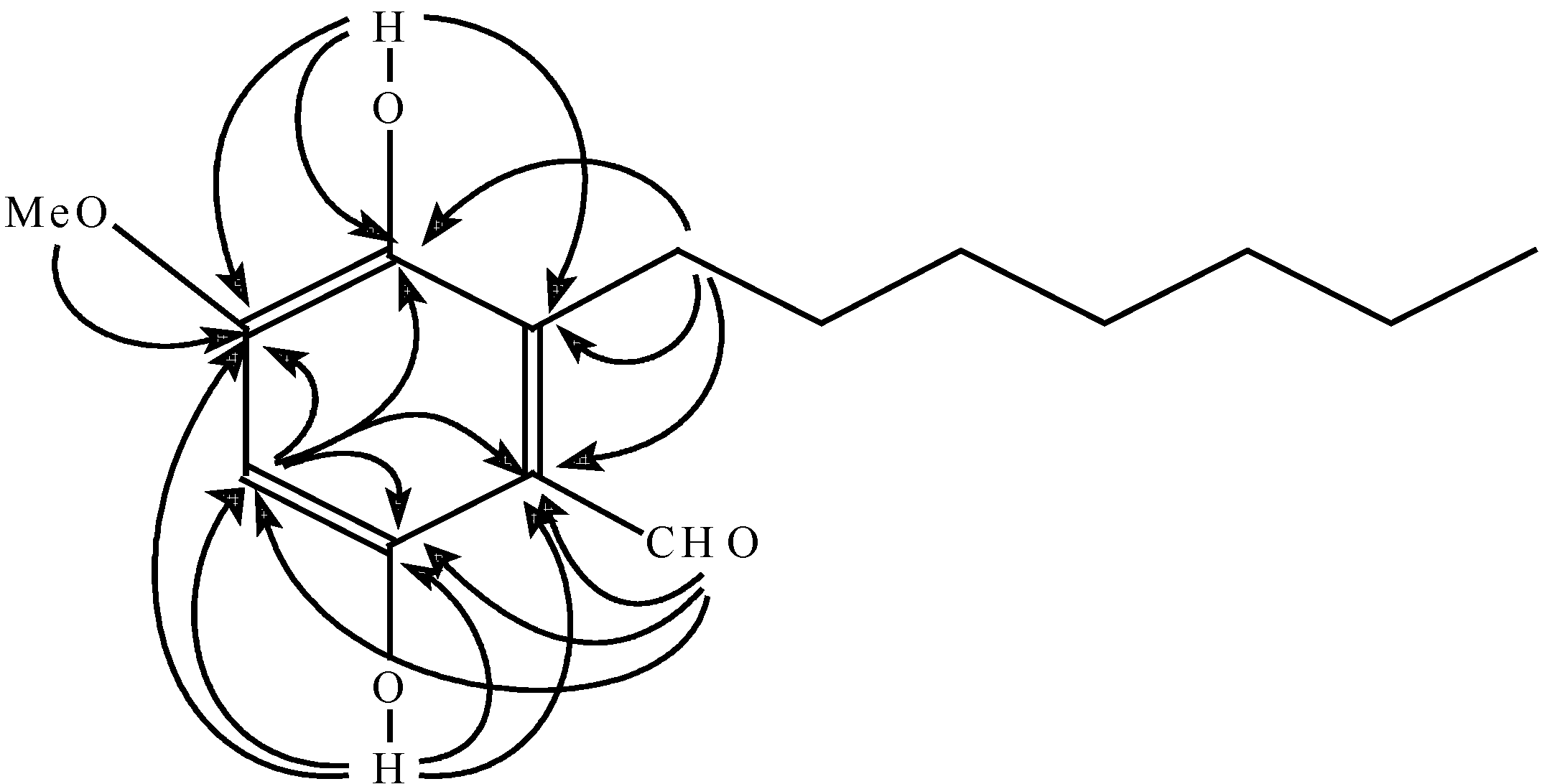
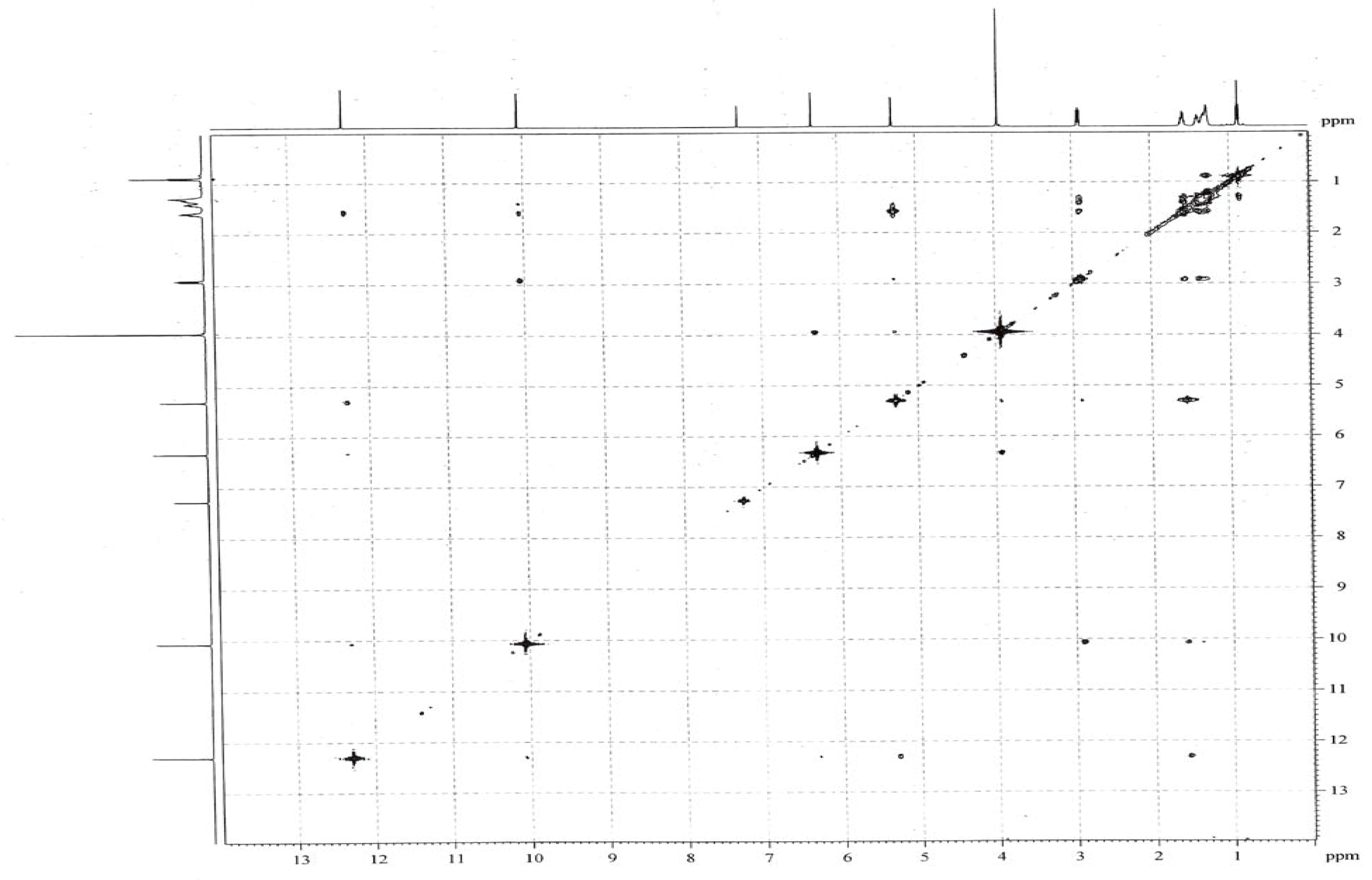
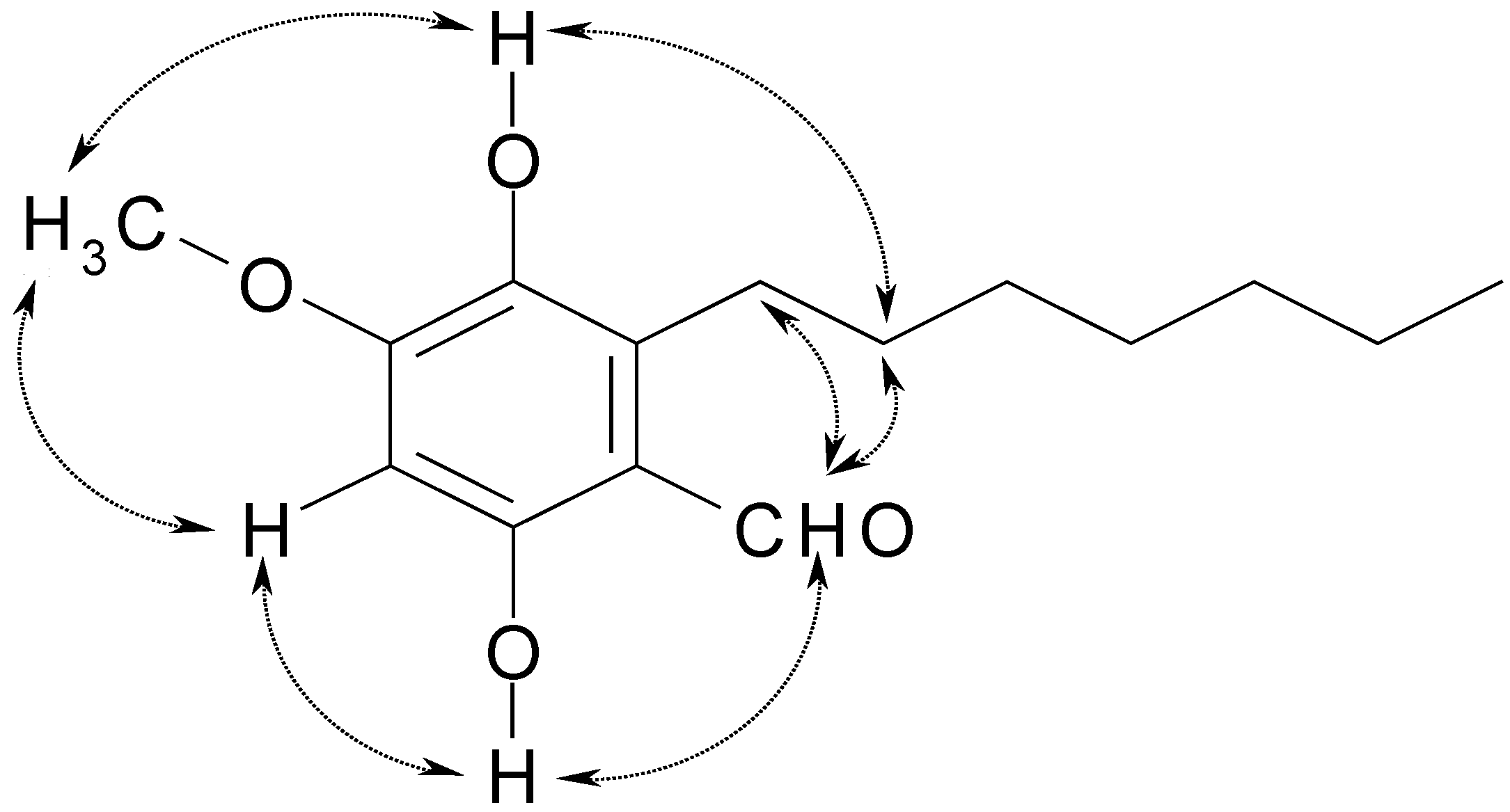
Conclusions
Experimental
General
Plant material
Extraction and isolation
| Position | 13C | 1H | HMBC |
|---|---|---|---|
| 1 | 111.6 | ||
| 2 | 129.8 | ||
| 3 | 136.1 | ||
| 4 | 154.1 | ||
| 5 | 97.0 | 6.30 (s, 1H) | 111.6, 136.1, 154.1, 159.9 |
| 6 | 159.9 | ||
| 1′ | 24.0 | 2.88 (t, 2H, J=8.0 Hz) | 29.5, 31.5, 111.6, 129.8, 136.1 |
| 2′ | 31.5 | 1.56 (m, 2H) | 24.0, 29.1 |
| 3′ | 29.5 | 1.35 (m, 2H) | 29.1 |
| 4′ | 29.1 | 1.28 (m, 2H) | 31.8 |
| 5′ | 31.8 | 1.28 (m, 2H) | |
| 6′ | 22.6 | 1.28 (m, 2H) | 31.8 |
| 7′ | 14.1 | 0.85 (t, 3H, J=6 Hz) | 22.6, 31.8 |
| 1-CHO | 193.4 | 10.03 (s, 1H) | 97.0, 111.6, 159.9 |
| 3-OH | 5.28 (s, 1H, exchangeable) | 129.8, 136.1, 154.1 | |
| 4-OCH3 | 56.2 | 3.90 (s, 3H) | 154.1 |
| 6-OH | 12.28 (s, 1H, exchangeable) | 97.0, 111.6, 154.1, 159.9 |
Cytotoxicity Assays
Acknowledgements
References
- Huang, X. A.; Yang, R. Z. A new hydroquinone diglucoside from Lysimachia fordiana. Chem. Nat. Compd. 2004, 40, 457–459. [Google Scholar] [CrossRef]
- Markham, K. R.; Ternal, B.; Stanley, R.; Geiger, H.; Mabry, T. J. Carbon-13 NMR studies of flavonoids-III. Tetrahedron 1978, 34, 1389–1397. [Google Scholar]
- Braun, S.; Kalinowski, H.-O.; Berger, S. 150 and More Basic NMRExperiments: A Practical Course; 2nd expanded ed.; VCH: Weinheim, 1998; pp. 460–463. [Google Scholar]
- Sample Availability: Available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Huang, X.-a.; Yang, R.-z.; Deng, W.-d. A New Poly-substituted Benzaldehyde from the Leaves of Lysimachia fordiana Oliv. Molecules 2007, 12, 43-48. https://doi.org/10.3390/12010043
Huang X-a, Yang R-z, Deng W-d. A New Poly-substituted Benzaldehyde from the Leaves of Lysimachia fordiana Oliv. Molecules. 2007; 12(1):43-48. https://doi.org/10.3390/12010043
Chicago/Turabian StyleHuang, Xin-an, Ren-zhou Yang, and Wen-di Deng. 2007. "A New Poly-substituted Benzaldehyde from the Leaves of Lysimachia fordiana Oliv." Molecules 12, no. 1: 43-48. https://doi.org/10.3390/12010043




