Synthesis, Chemical Characterization and Biological Screening for Cytotoxicity and Antitumor Activity of Organotin (IV) Derivatives of 3,4-Methylenedioxy 6-nitrophenylpropenoic Acid
Abstract
:Introduction
Results and Discussion
Synthesis
 RSn(Cl)L2 + 2H2O
RSn(Cl)L2 + 2H2O
R= n-Bu (3)
 R2SnL2 + H2O
R2SnL2 + H2O
R = n-Bu (4), n-Oct (6)
 R2SnL2 + 2NaCl
R2SnL2 + 2NaCl
R = Me (1), Et (2)
 R3SnL + NaCl
R3SnL + NaCl
R = n-Bu (5)
 R3SnL+H2O
R3SnL+H2O
R= Cy (7), Ph (8)
| Compound No. | Molecular Formula | Molecular Weight | M.P. (˚C) | Yield(%) | Elemental Analysis
% Calculated (Found) | ||
|---|---|---|---|---|---|---|---|
| C | H | N | |||||
| 1 | C22H18O12N2Sn | 622 | 237-240 | 86 | 42.51 (42.48) | 2.90 (2.96) | 4.51 (4.58) |
| 2 | C24H22O12N2Sn | 650 | 176-178 | 67 | 44.35 (44.40) | 3.46 (3.43) | 4.31 (4.42) |
| 3 | C24H26O12N2SNCl | 689.5 | 142-145 | 70 | 42.13 (42.24) | 3.07 (3.15) | 4.10 (4.04) |
| 4 | C28H30O12N2Sn | 706 | 196-199 | 73 | 47.66 (47.74) | 4.26 (4.16) | 3.97 (4.01) |
| 5 | C22H33O6NSn | 527 | 143-146 | 89 | 50.19 (50.23) | 6.27 (6.30) | 2.66 (2.70) |
| 6 | C36H46O12N2Sn | 818 | 151-153 | 75 | 52.88 (52.96) | 5.63 (5.60) | 3.43 (3.50) |
| 7 | C28H39O6NSn | 605 | 99-101 | 83 | 35.63 (35.69) | 6.46 (6.50) | 2.32 (2.36) |
| 8 | C28H21O6NSn | 587 | 207-210 | 80 | 57.34
(54.43) | 3.58
(3.60) | 2.39
(2.45) |
| Ligand | C10H7O6N | 237 | 278 | 80 | 50.63 (50.45) | 2.95 (2.80) | 5.90 (5.87) |
1H-NMR Spectroscopy
| 1H No | Compound | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligand | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| 2 | 7.09 (s) | 7.12 (s) | 7.00 (s) | 7.10 (s) | 7.09 (s) | 7.01 (s) | 7.02 (s) | 7.03 (s) | 6.96 (s) |
| 5 | 7.52 (s) | 7.58(s) | 7.54 (s) | 7.58 (s) | 7.52 (s) | 7.51 (s) | 7.33 (s) | 7.51(s) | 7.78 (s) |
| 7 | 6.29 (s) | 6.24 (s) | 6.12 (s) | 6.00 (s) | 6.11 (s) | 6.14 (s) | 5.98 (s) | 6.14 (s) | 6.11 (s) |
| 8 | 7.82 (d,15.9) | 7.96 (d,15.0) | 8.17 (d,16.5) | 7.95 (d,15.0) | 7.99 (d, 15.0) | 8.00 (d,15.0) | 7.82 (d,16.0) | 8.02 (d, 15.5) | 7.82 (d,16.0) |
| 9 | 6.50 (d,15.6) | 6.47 (d,15.0) | 6.33 (d,15.0) | 6.47 (d,15.0) | 6.30 (d, 15.0) | 6.31 (d,15.0) | 6.17 (d,15.5) | 6.31 (d, 15.5) | 6.30 (d,15.0) |
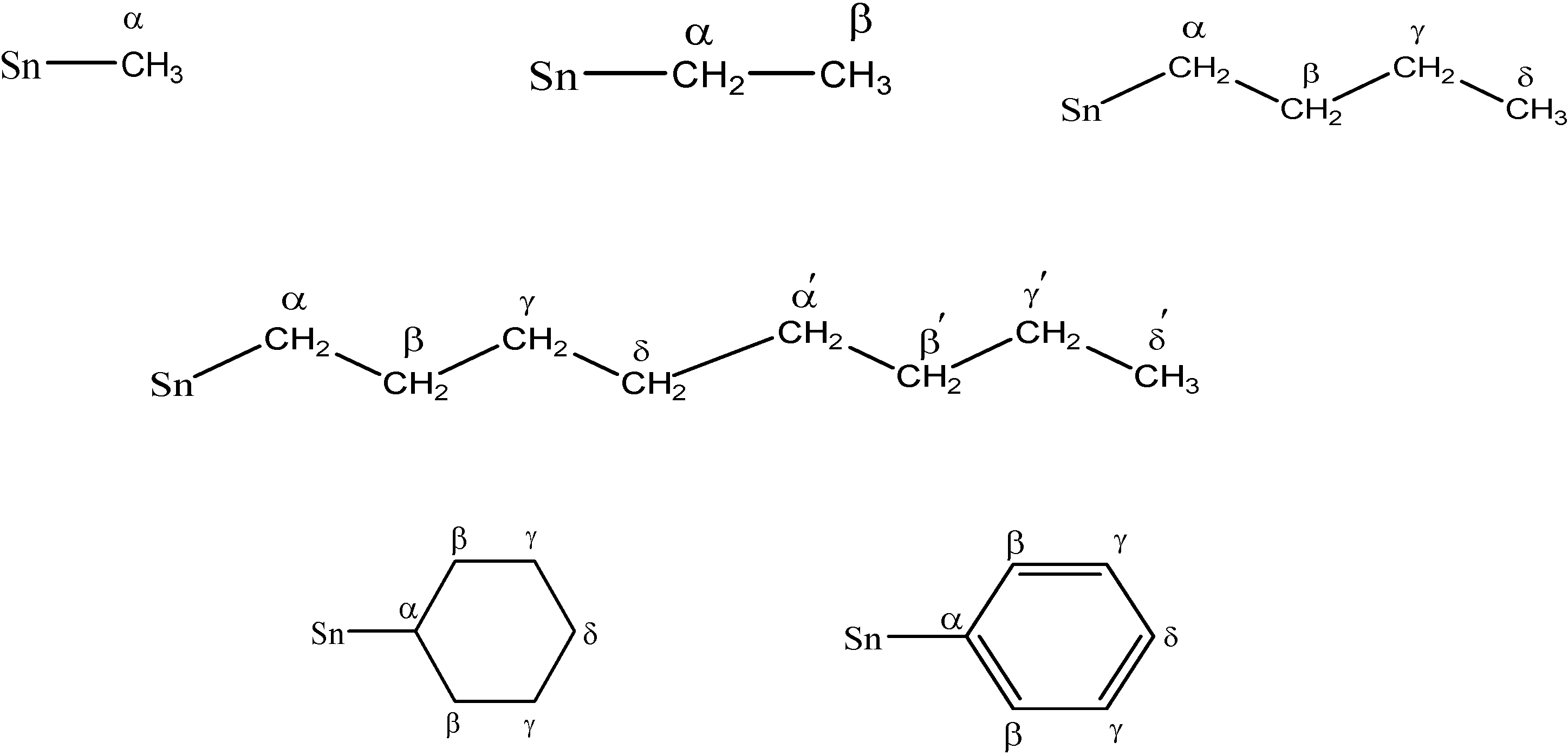
| α | − | 0.89 (s) | 1.52 (q, 10.0) | − | 1.72 (t, 8.0) | 1.6 | 1.49-1.53 (m) | − | − |
| β | − | − | 1.34 (t, 8.2) | − | 1.34-1.37 (m) | 1.61-1.65 (m) | 1.49-1.53 (m) | 1.26-1.76 (m) | 7.35-7.49 (m) |
| γ | − | − | − | − | 1.34-1.37 (m) | 1.33-1.37 (m) | − | 1.26-1.76 (m) | 7.35-7.49 (m) |
| δ | − | − | − | − | 0.91 (t, 10.0) | 0.92 (t, 8.5) | − | 1.26-1.76 (m) | 7.35-7.49 (m) |
| γ-γ′ | − | − | − | − | − | − | 1.15 (bs) | − | − |
| δ' | − | − | − | − | − | − | 0.65 (t, 7.0) | − | − |

| Compound No. | 1J[119Sn, 13C] (Hz) | 2J[119Sn, 1H] (Hz) | Angle (o) | |
|---|---|---|---|---|
| 1J | 2J | |||
| 1 | - | 78 | - | 128.4 |
| 2 | - | 72 | - | 121.8 |
| 3 | - | - | - | - |
| 4 | - | - | - | - |
| 5 | 360 | 74 | 108.33 | 123.9 |
| 6 | - | - | - | - |
| 7 | - | - | - | - |
| 8 | - | - | - | - |
13C-NMR Spectroscopy
| 13 CNo | Ligand | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 107.19 | 105.93 | 107.64 | 107.65 | 107.46 | 107.37 | 107.68 | 107.39 | 107.40 |
| 3 | 151.73 | 153.10 | 152.05 | 152.62 | 151.90 | 151.90 | 153.09 | 151.87 | 151.94 |
| 4 | 148.73 | 150.18 | 149.24 | 148.78 | 148.65 | 148..63 | 150..29 | 148.55 | 148.87 |
| 5 | 106.90 | 104.86 | 105.18 | 105.01 | 105.60 | 105.56 | 105.75 | 105.56 | 105.63 |
| 6 | 138.87 | 141.35 | 142.67 | 143.81 | 138.96 | 139.03 | 141.52 | 138.78 | 140.88 |
| 7 | 103.74 | 104.30 | 103.06 | 103.45 | 103.26 | 103.25 | 104.68 | 103.20 | 103.20 |
| 8 | 142.98 | 141.25 | 143.92 | 143.81 | 143.13 | 143.06 | 144.34 | 143.07 | 143.10 |
| 9 | 122.67 | 123.10 | 120.78 | 124.54 | 125.82 | 124.54 | 122.49 | 124.85 | 122.77 |
| 10 | 167.03 | 187.87 | 175.39 | 186.84 | 171.62 | 170.90 | 174.75 | 170.70 | 171.96 |
| α | - | 4.34 | 19.17 | 22.70 | 22.70 | 15.24 [340, 360] | 25.43 | 14.11 | 130.22 |
| β | - | - | 14.11 | 25.57 | 26.37 | 26.79 | 23.27 | 29.37 | 137.13 |
| γ | - | - | - | 29.19 | 26.83 | 27.04 | 33.56 | 31.95 | 128.97 |
| δ | - | - | - | 14.11 | 13.67 | 13.64 | 29.72 | 28.85 | 128.97 |
| α′ | - | - | - | - | - | - | 31.34 | - | - |
| β′ | - | - | - | - | - | - | 30.29 | - | - |
| γ′ | - | - | - | - | - | - | 32.51 | - | - |
| δ′ | - | - | - | - | - | - | 25.90 | - | - |
| 119 Sn | - | -112.63 | -150.68 | -150.03 | -156.43 | +116.10 | -175.00 | 45.64 | -112.45 |
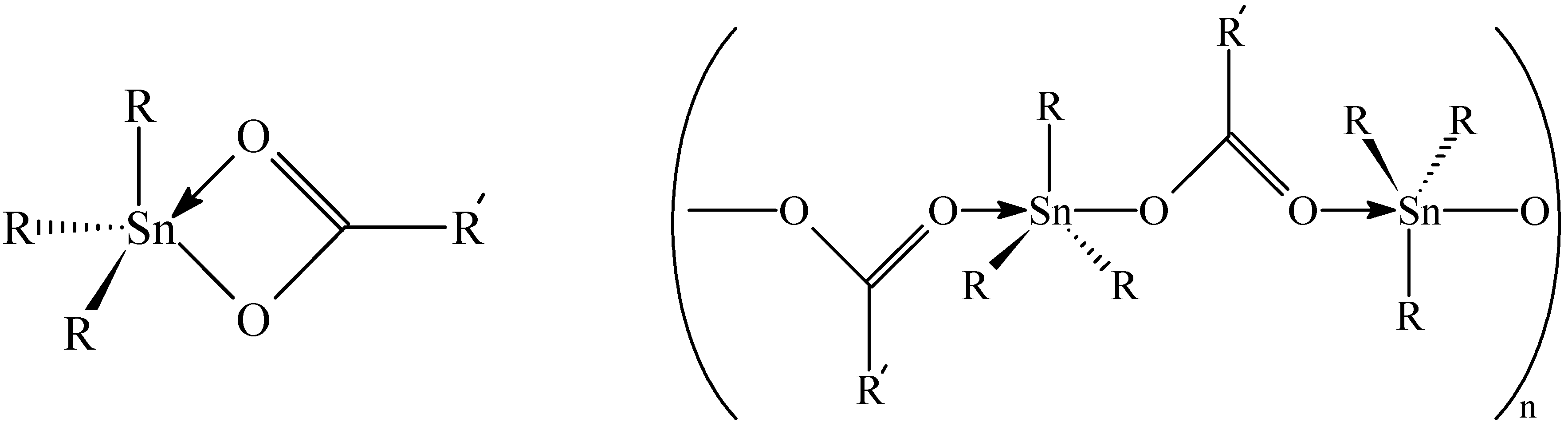
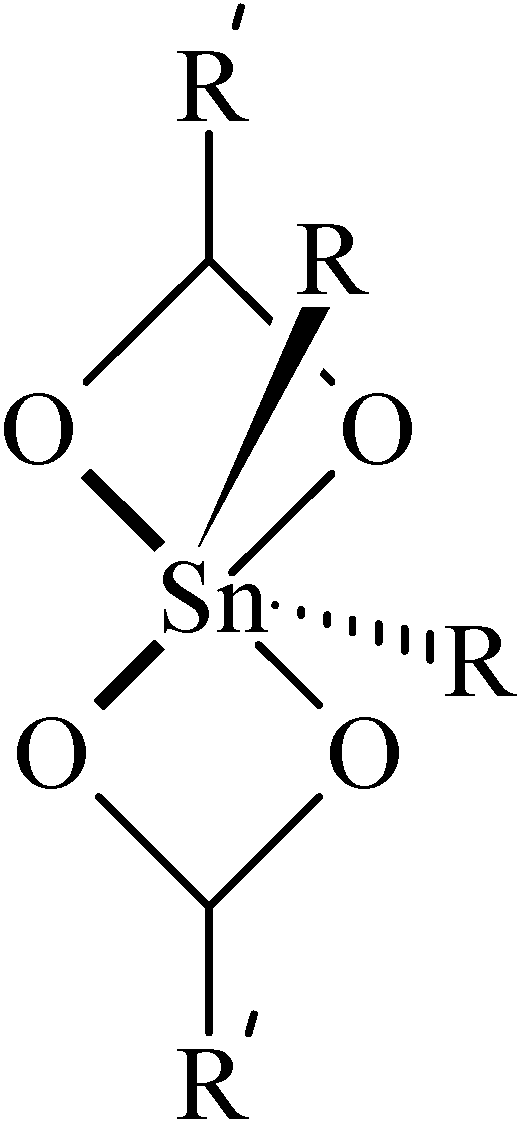
119Sn-NMR Spectroscopy
Mass Spectrometry
| Fragment, m/z (%) | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| [C23H20N2O12Sn]+ 636 (8.5) | [C22H16N2O12Sn]+ 620 (3.5) | [C24H19ClN2O12Sn]+ 682.5 (2.5) | [C32H37N2O12Sn]+ 761(16.5) | [C30H50NO6Sn]+ 640(7.3) | [C40H54N2O12Sn]+ 874 (9.5) | [C28H39NO6Sn]+ 605 (2.5) | [C22H15NO6Sn]+ 509(14.9) |
| [C22H16N2O12Sn]+ 620 (17.3) | [C15H12NO8Sn]+ 454 (41.6) | [C20H10ClN2O12Sn]+ 625.5 (11.3) | [C27H26N2O12Sn]+ 690 (7.6) | [C22H31NO6Sn]+ 525 (6.4) | [C34H40N2O12Sn]+ 788 (16.2) | [C27H36NO4Sn]+ 558 (7.3) | [C21H13NO4Sn]+ 463 (3.3) |
| [C21H15N2O10Sn]+ 575 (3.4) | [C10H13O4Sn]+ 317 (17.1) | [C14H14Cl NO6Sn]+ 447.5 (32.7) | [C24H19N2O12Sn]+ 647 (19.9) | [C18H23 NO6Sn]+ 469 (23.6) | [C28H28N2O10Sn]+ 672 (26.3) | [C22H27NO6Sn]+ 521 (33.4) | [C15H8 NO4Sn]+ 386 (27.1) |
| [C19H9N2O10Sn]+ 545 (37.7) | [Sn]+ 120 (3.7) | [C5H11Sn]+ 191 (41.5) | [C8H17N2O12Sn]+ 453 (25.2) | [C14H14NO6Sn]+ 412 (12.7) | [C18H22NO6Sn]+ 468 (8.7) | [C16H16NO6Sn]+ 438 (17.1) | [C9H6O2Sn]+ 266 (72.4) |
| [C11H6NO6Sn]+ 368 (60.9) | [C4H9]+ 57 (100) | [C4H8Sn]+ 176 (6.9) | [C4H7Sn]+ 175 (79.7) | [C7H10O2Sn]+ 246 (40.7) | [C9H16O2Sn]+ 276 (100) | [C9H12O2Sn]+ 272 (63.4) | [C11H6NO6Sn]+ 368 (60.9) |
| [C4H4O2Sn]+ 204 (100) | - | [C3H8Sn]+ 164 (18.8) | [C2H3Sn]+ 145 (100) | [C4H9]+ 57 (100) | [C4H9]+ 57 (76.3) | [C4H6O2Sn]+ 206 (100) | [C6H5]+ 77 (100) |
| - | - | [C4H9]+ 57 (100) | - | - | - | - | - |
Biological Activity
Cytotoxicity
| Compound No | LD50 µg/mL |
|---|---|
| 1 | - |
| 2 | 130.98 |
| 3 | 581.23 |
| 4 | 33.39 |
| 5 | 0.26 |
| 6 | 191.64 |
| 7 | 0.40 |
| 8 | 0.56 |
| Ligand | 975.24 |
Antitumor Activity
| Compound No. | Average number of tumors ± SE | % inhibition of tumors |
|---|---|---|
| 1 | 2.8±0.89 | 62.66 |
| 2 | 0.0±0.0 | 100 |
| 3 | 3.9±1.02 | 48 |
| 4 | 1.0±0.36 | 86.66 |
| 5 | 0.0±0.0 | 100 |
| 6 | 5.3±0.84 | 29.33 |
| 7 | 4.8±1.13 | 36 |
| 8 | 0.0±0.0 | 100 |
| Ligand | 4.02±1.04 | 44 |
| (-)ive control | 7.5±1.13 | - |
Experimental
General
Synthesis
Synthesis of the Ligand L
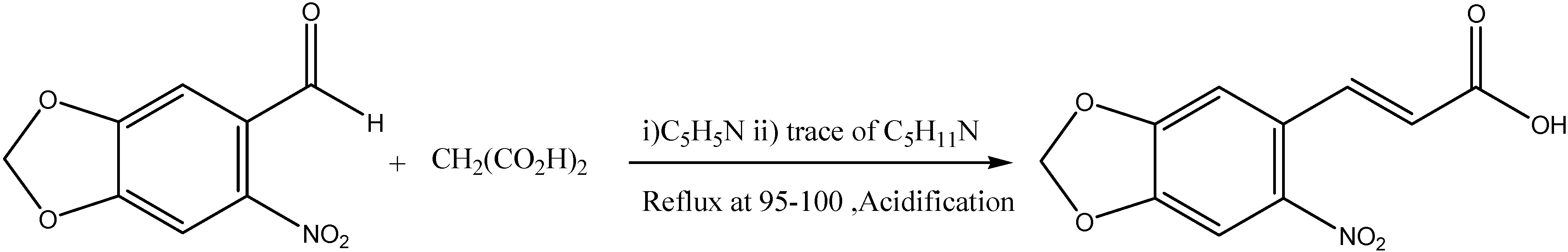
Synthesis of Complexes
Procedure-I
Procedure-II
Biological Activity
Cytotoxicity
Antitumor Activity
Acknowledgments
References
- Davies, A. G. Organotin Chemistry; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Gielen, M. An overview of forty years organotin chemistry developed at the Free Universities of Brussels ULB and VUB. J. Braz Chem. Soc. 2003, 14, 870–877. [Google Scholar] [CrossRef]
- Casini, A.; Messori, L.; Orioli, P.; Gielen, M.; Kemmer, M.; Willem, R. Interactions of two cytotoxic organotin (IV) compounds with calf thymus DNA. J. Inorg. Biochem. 2001, 85, 297–300. [Google Scholar] [CrossRef]
- Gielen, M.; Handlir, K.; Hollein, M.; De, Vos. D. Synthesis, Characterization and Antitumour Activity of Some Butyltin (IV) Cysteaminates and N,N-Dimethylcysteaminates. Met. Based Drugs. 2000, 7, 233–236. [Google Scholar] [CrossRef]
- Gielen, M.; Biesemans, M.; De-Vos, D.; Willem, R. Synthesis, characterization and in vitro antitumor activity of di- and triorganotin derivatives of polyoxa- and biologically relevant carboxylic acids. J. Inorg. Biochem. 2000, 79, 139–45. [Google Scholar] [CrossRef]
- Bonire, J. J.; Fricker, S. P. The in vitro antitumour profile of some 1, 2-diaminocyclohexane organotin complexes. J. Inorg. Biochem. 2001, 83, 217–220. [Google Scholar] [CrossRef]
- De-Vos, D.; Willem, R.; Gielen, M.; Van, Wingerden. K.E.; Nooter, K. The Development of Novel Organotin Anti-Tumor Drugs: Structure and Activity. Met. Based Drugs. 1998, 5, 179–188. [Google Scholar]
- Gielen, M.; Biesemans, M.; Willem, R. Organotin compounds: from kinetics to stereochemistry and antitumor activities. Appl. Organomet. Chem. 2005, 19, 440–450. [Google Scholar] [CrossRef]
- Yin, D. H.; Wang, B. Q.; Xing, J. Q. Synthesis and characterization of trialkyltin esters of pyridinecarboxylic acids and crystal structure of [n Bu3SnO2CC5H4N-2] ∞. Heteroatom Chem. 2004, 15, 524–529. [Google Scholar]
- Ali, S.; Ahmad, F.; Mazhar, M.; Munir, A.; Masood, M. T. Synthesis and Spectral Studies of Di- and Triorganotin (IV) Complexes with 2-(6-Methoxynaphthyl) propionic Acid (Naproxen). Synth. React. Inorg. Met-Org. Chem. 2001, 32, 357–372. [Google Scholar]
- Armarego, W. L. F.; Chai, C. L. L. Purification of Laboratory Chemicals, 5th Ed ed; Butterworth-Heinemann: London, 2004. [Google Scholar] Rehman, A.; Choudhary, M. I. Bioassay Techniques for Drug Development; Harwood Academic Publishers: Amsterdam, 2001; pp. 9–14. [Google Scholar]
- Masood, M. T.; Ali, S.; Danish, M.; Mazhar, M. Synthesis and Characterization of Tri-, Di-, and Chlorodiorganotin (IV) Derivatives of 3-Benzoyl-alpha-methylphenylacetic Acid and 3-(2-Thienyl)acrylic Acid. Synth. React. Inorg. Met-Org. Chem. 2002, 32, 9–24. [Google Scholar] Geilen, M.; Meunier-Piret, J.; Biesemans, M.; Willem, R.; Khloufo, A. El. Synthesis, spectroscopic characterization and in vitro antitumour activity of di-n-butyltin and diethyltin trimethoxybenzoates: X-ray structure analysis of bis[di-n-butyl(3,4,5-trimethoxybenzoato)tin] oxide. Appl. Organomet. Chem. 1992, 6, 67–69. [Google Scholar]
- Lockhart, T. P.; Manders, W. F. Structure determination by NMR spectroscopy. Correlation of |[2]J([119]Sn,[1]H)| and the Me-Sn-Me angle in methyltin (IV) compounds. Inorg. Chem. 1986, 25, 892–298. [Google Scholar] [CrossRef]
- Nadvornik, M.; Holeček, J.; Handlir, K.; Lycka, A. The [13]C and [119]Sn NMR spectra of some four- and five-coordinate tri-n-butyltin (IV) compounds. J. Organomet. Chem. 1984, 275, 43–51. [Google Scholar]
- Shahzadi, S.; Bhatti, M. H.; Shahid, K.; Ali, S.; Tariq, S. R.; Mazhar, M.; Khan, K. M. Synthesis, Characterization, and Biological Activity of n-Tributyltin Derivatives of Pharmaceutically Active Carboxylates. Monatsh. Chem. 2002, 133, 1089–1096. [Google Scholar] [CrossRef]
- Ahmed, F.; Ali, S.; Parvez, M.; Munir, A.; Mazhar, M.; Shah, T. A. Synthesis, characterization, and biological studies of tri- and diorganotin (IV) complexes with 2,4-difluoro-4-hydroxy-[1,1]-biphenyle-3-carbolic acid: Crystal structure of [(CH3)3Sn(C13H7O3F2)]. Heteroatom. Chem. 2002, 13, 638–642. [Google Scholar] [CrossRef]
- Danish, M.; Ali, S.; Mazhar, M.; Badshah, A.; Choudhary, M. I.; Alt, H. G.; Kehr, G. Moessbauer, multinuclear magnetic resonance and mass spectrometric studies of organotin carboxylates of m-methyl trans-cinnamic acid. Polyhedron 1995, 14, 3115–3120. [Google Scholar] [CrossRef]
- Shahid, K.; Ali, S.; Shahzadi, S.; Badshah, A.; Khan, K. M.; Maharvi, G. M. Organotin (IV) Complexes of Aniline Derivatives. I. Synthesis, Spectral, and Antibacterial Studies of Di- and Triorganotin (IV) Derivatives of 4-Bromomaleanilic Acid. Synth. React. Inorg. Met.-Org. Chem. 2003, 33, 1221–1236. [Google Scholar] [CrossRef]
- Sadiq-ur-Rehman; Ali, S; Mazhar, M; Badshah, A; Parvez, M. Synthesis, spectroscopic characterization, and biological activity studies of organotin (IV) derivatives of (E)-3-(3-fluorophenyl)-2-phenyl-2-propenoic acid. Crystal and molecular structure of Et2Sn[OCOC(C6H5) CH(3-FC6H4)]. Heteroatom Chem. 2006, 17, 420–432. [Google Scholar]
- Holeček, J.; Lycka, A. Dependence of [[1] J ([119] Sn [13] C)] on the C―Sn―C angle in n-butyltin (IV) compounds. Inorg. Chim. Acta. 1986, 118, L15–L16. [Google Scholar] [CrossRef]
- Kapoor, R; Gupta, A; Kapoor, P; Venugopalan, P. Synthesis and characterization of di-n-butyltin (IV) complexes with 4′/2′-nitrobiphenyl-2-carboxylic acids: X-ray crystal structures of [{(n-C4H9)2Sn(OCOC12H8NO2-4′)}2O]2 and (n-C4H9)2Sn{OCOC12H8NO2-4′}2. Appl. Organomet. Chem. 2003, 17, 600–606. [Google Scholar] [CrossRef]
- Holeček, J.; Nadvornik, M.; Handlir, K.; Lycka, A. 13C and 119Sn NMR spectra of di-n-butyltin (IV) compounds. J. Organomet. Chem. 1986, 315, 299–308. [Google Scholar] [CrossRef] Tiekink, E. R. T.; Gielen, M.; Bouhbid, A. Crystal structure of the dimeric bis(p-fluoro- and pentafluorophenylacetato)tetra-n-butyldistannoxanes. J. Organomet. Chem. 1995, 494, 247–253. [Google Scholar] [CrossRef]
- Tian, L; Yu, Q; Zheng, X; Shang, Z; Xueli, Liu; Qian, B. Synthesis, Characterization and in vitro anitumour activity of di- and tri-organotin derivatives of fenbufen. Appl. Organomet. Chem. 2005, 19, 672–676. [Google Scholar] [CrossRef]
- Mala, N.; Sulaxna; Song, X; Eng, G. Synthesis, spectral and thermal studies of some organotin (IV) derivatives of 5-amino-3H-1,3,4-thiadiazole-2-thione. Spectrochim. Acta A 2006, 64, 148–155. [Google Scholar] [CrossRef]
- Williams, D. H.; Fleming, I. Spectroscopic Methods in Organic Chemistry, 4th Edn. ed; McGraw-Hill Book Company (UK) Ltd.: London, 1987; p. 136. [Google Scholar]
- Ali, S.; Khokhar, M. N.; Bhatti, M. H.; Mazhar, M.; Masood, M. T.; Shahid, K.; Badshah, A. 1H, 13C, and 119Sn NMR, IR, Mass, Thermal, and Biological Studies of Organotin (IV) Derivatives of 4-p-(Chlorophenyl)-2-phenyl-5-thiazoleacetic Acid. Synth. React. Inorg. Met.-Org. Chem. 2002, 32, 1373–1392. [Google Scholar] [CrossRef]
- Sadiq-Ur-Rehman; Ali, S.; Badshah, A.; Malik, A.; Ahmed, V.; Jin, G. X.; Edward , R. T.; Tiekink. Di- and tri-organotin (IV) derivatives of (Z)-3-(4-nitrophenyl)-2-phenyl-2-propenoic acid: spectroscopic characterization and biocidal studies. Crystal structure analysis of tetrameric tri-n-butyltin (IV) (Z)-3-(4-nitrophenyl)-2-phenyl-2-propenoate. Appl. Organomet. Chem. 2004, 18, 401–408. [Google Scholar] [CrossRef]
- Danish, M.; Alt, H. G.; Badshah, A.; Ali, S.; Mazhar, M.; Islam, N. Organotin esters of 3-(2-furanyl)-2-propenoic acid: their characterization and biological activity. J. Organomet. Chem. 1995, 486, 51–56. [Google Scholar] [CrossRef]
- Sample Availability: Contact the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Ahmad, M.S.; Hussain, M.; Hanif, M.; Ali, S.; Mirza, B. Synthesis, Chemical Characterization and Biological Screening for Cytotoxicity and Antitumor Activity of Organotin (IV) Derivatives of 3,4-Methylenedioxy 6-nitrophenylpropenoic Acid. Molecules 2007, 12, 2348-2363. https://doi.org/10.3390/12102348
Ahmad MS, Hussain M, Hanif M, Ali S, Mirza B. Synthesis, Chemical Characterization and Biological Screening for Cytotoxicity and Antitumor Activity of Organotin (IV) Derivatives of 3,4-Methylenedioxy 6-nitrophenylpropenoic Acid. Molecules. 2007; 12(10):2348-2363. https://doi.org/10.3390/12102348
Chicago/Turabian StyleAhmad, Muhammad Sheeraz, Mukhtiar Hussain, Muhammad Hanif, Saqib Ali, and Bushra Mirza. 2007. "Synthesis, Chemical Characterization and Biological Screening for Cytotoxicity and Antitumor Activity of Organotin (IV) Derivatives of 3,4-Methylenedioxy 6-nitrophenylpropenoic Acid" Molecules 12, no. 10: 2348-2363. https://doi.org/10.3390/12102348




