Reactive Oxygen Species Scavenging Activity of Flavone Glycosides from Melilotus neapolitana
Abstract
:Introduction
Results and Discussion
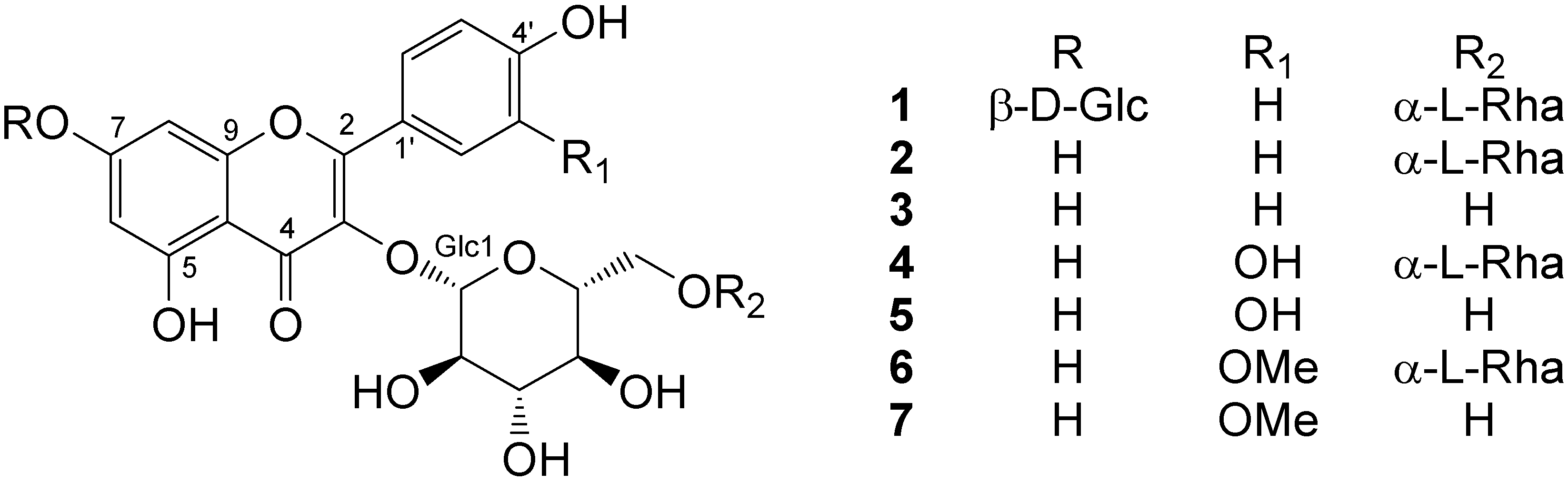
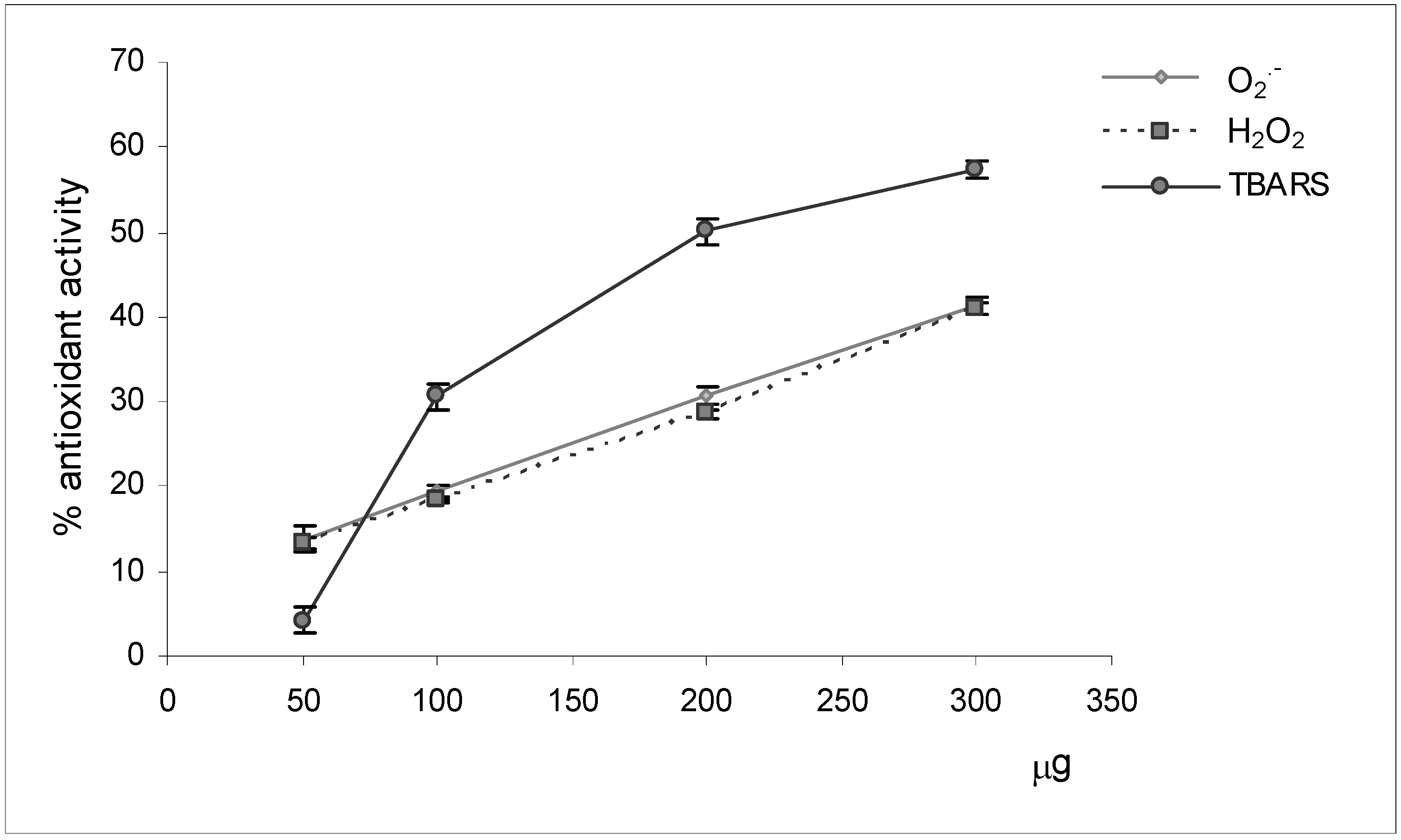
Reactive oxygen species scavenging activities
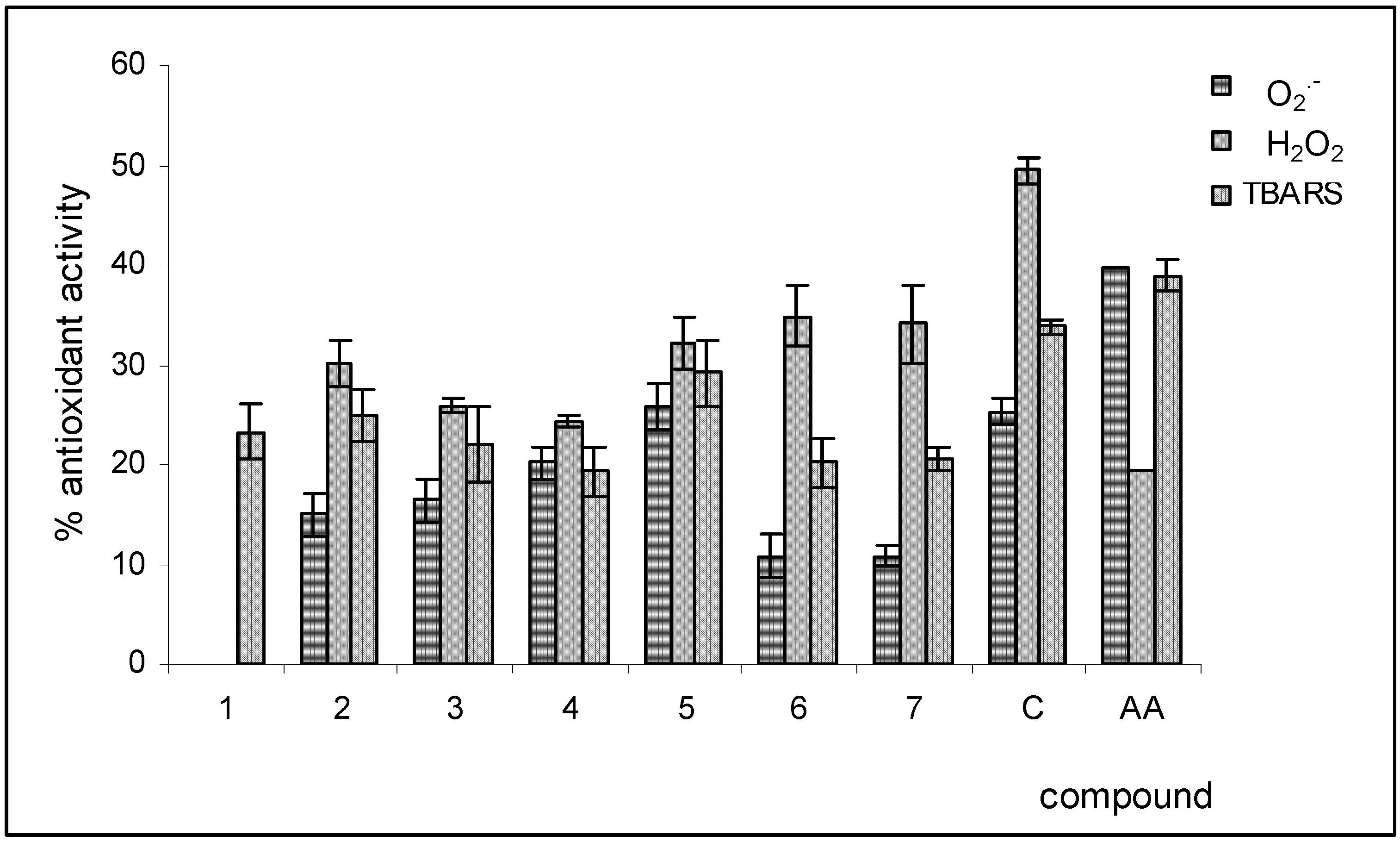
Conclusions
Experimental Section
General
Plant material
Extraction and isolation
O2.- scavenging activity
H2O2 scavenging activity
Determination of TBARS
References
- Dajas, F.; Arredondo, F.; Echeverry, C.; Ferreira, M.; Morquio, A.; Rivera, F. Flavonoids and the brain: Evidences and putative mechanisms for a protective capacity. Curr. Neuropharmacol. 2005, 3, 193–205. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Tosic, M.; Marjanovitz, B.; Simic, M.G. Flavonoids as antioxidants. J. Am.Chem. Soc. 1994, 116, 4846–4851. [Google Scholar] [CrossRef]
- Hertog, M.G.; Feskens, E.J.; Kromhout, D. Antioxidant flavonols and coronary heart disease risk. Lancet 1997, 349, 699. [Google Scholar]
- Cotelle, N.; Bernier, J.L.; Catteau, J.P.; Pommery, J.; Wallet, J.C.; Gaydou, E.M. Antioxidant properties of hydroxy-flavones. Free Radic. Biol. Med. 1996, 20, 35–43. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Fiorentino, A.; Oriano, P.; Monaco, P.; Pacifico, S. Annurcoic acid: a new antioxidant ursane triterpene from fruits of cv. Annurca apple. Food Chem. 2006, 98, 285–290. [Google Scholar] [CrossRef]
- Cefarelli, G.; D’Abrosca, B.; Fiorentino, A.; Izzo, A.; Mastellone, C.; Pacifico, S.; Piscopo, V. Free radical scavenging and antioxidant activities of secondary metabolites from reddened cv. Annurca apple fruits. J. Agric. Food Chem. 2006, 54, 803–809. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. The polyphenol constituents of grape pomace. Food Chem. 1999, 65, 1–8. [Google Scholar] [CrossRef]
- D'Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Monaco, P.; Previtera, L.; Simonet, A. M.; Zarrelli, A. Potential allelochemicals from Sambucus nigra. Phytochemistry 2001, 58, 1073–1081. [Google Scholar] [CrossRef]
- Yeskaliyeva, B.; Mesaik, M.A.; Abbaskhan, A.; Kulsoom, A.; Burasheva, G.Sh.; Abilov, Zh.A.; Choudhary, M.I.; Atta-ur-Rahman. Bioactive flavonoids and saponins from Climacoptera obtusifolia. Phytochemistry 2006, 67, 2392–2397. [Google Scholar] [CrossRef]
- Dasgupta, N.; De, B. Antioxidant activity of Piper betle L. leaf extract in vitro. Food Chem. 2004, 88, 219–224. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and antiradical activity of some phenolics acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compounds 2–7 are available from the authors.
© 2007 by MDPI (www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Fiorentino, A.; D'Abrosca, B.; Pacifico, S.; Golino, A.; Mastellone, C.; Oriano, P.; Monaco, P. Reactive Oxygen Species Scavenging Activity of Flavone Glycosides from Melilotus neapolitana. Molecules 2007, 12, 263-270. https://doi.org/10.3390/12020263
Fiorentino A, D'Abrosca B, Pacifico S, Golino A, Mastellone C, Oriano P, Monaco P. Reactive Oxygen Species Scavenging Activity of Flavone Glycosides from Melilotus neapolitana. Molecules. 2007; 12(2):263-270. https://doi.org/10.3390/12020263
Chicago/Turabian StyleFiorentino, Antonio, Brigida D'Abrosca, Severina Pacifico, Annunziata Golino, Claudio Mastellone, Palma Oriano, and Pietro Monaco. 2007. "Reactive Oxygen Species Scavenging Activity of Flavone Glycosides from Melilotus neapolitana" Molecules 12, no. 2: 263-270. https://doi.org/10.3390/12020263



