Methyl Jasmonate and Salicylic Acid Induced Oxidative Stress and Accumulation of Phenolics in Panax ginseng Bioreactor Root Suspension Cultures
Abstract
:Introduction
Results

| Duration | FW (g L-1) | DW (g L-1) | FW (g L-1) | DW (g L-1) |
| 0 day | 20.21a ± 2.11 | 4.34a ± 0.06 | 20.21a ± 0.16 | 4.34a ± 0.48 |
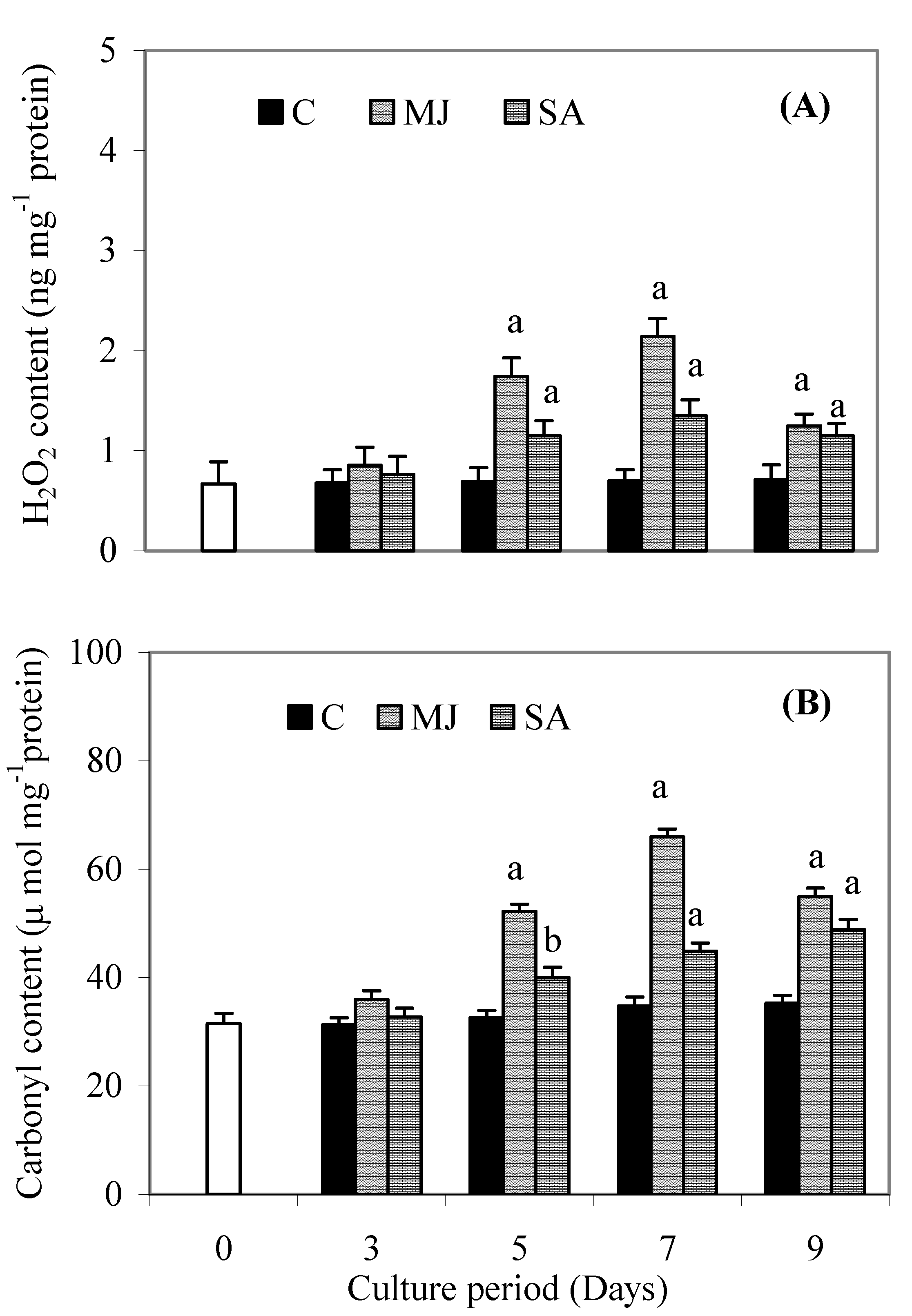
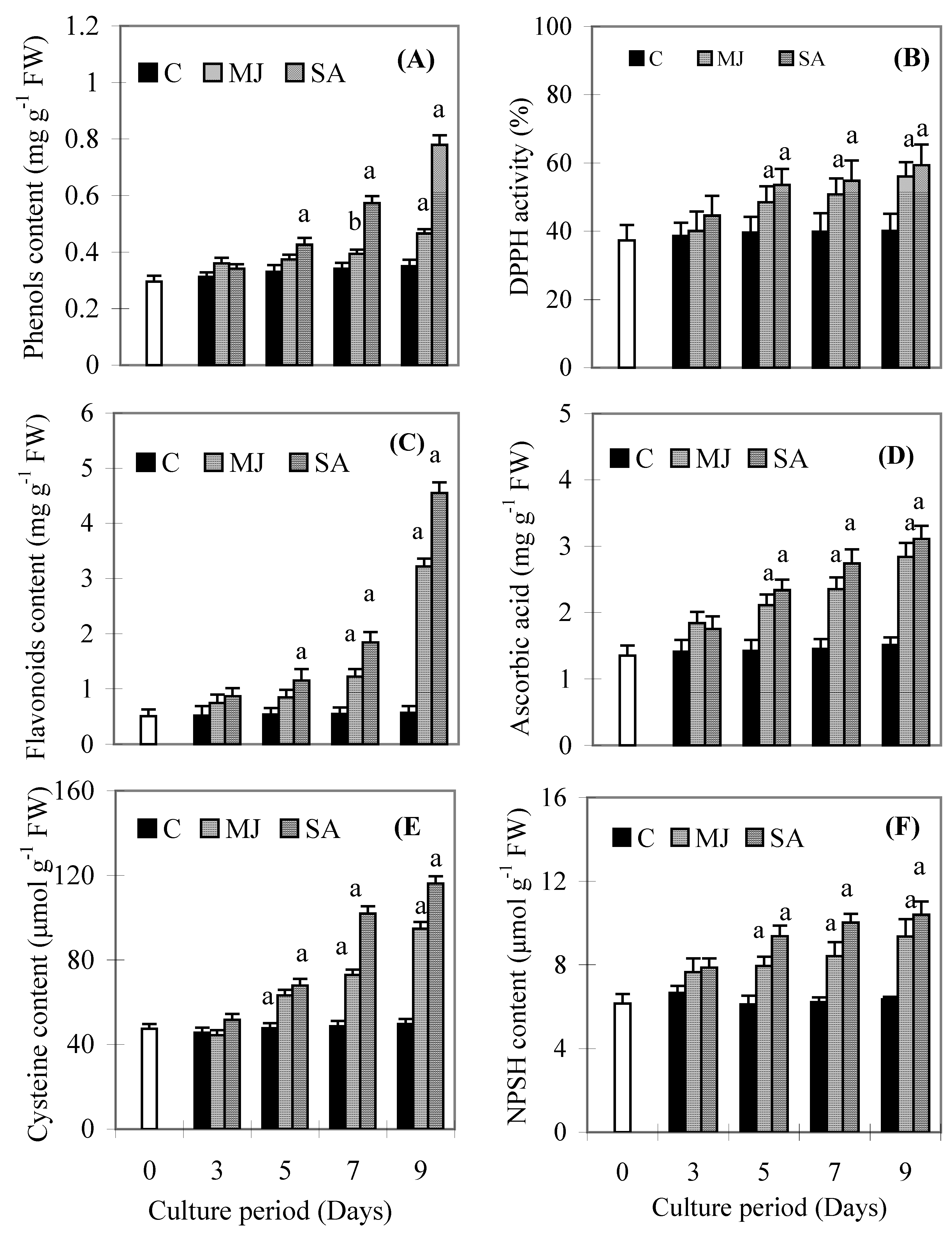
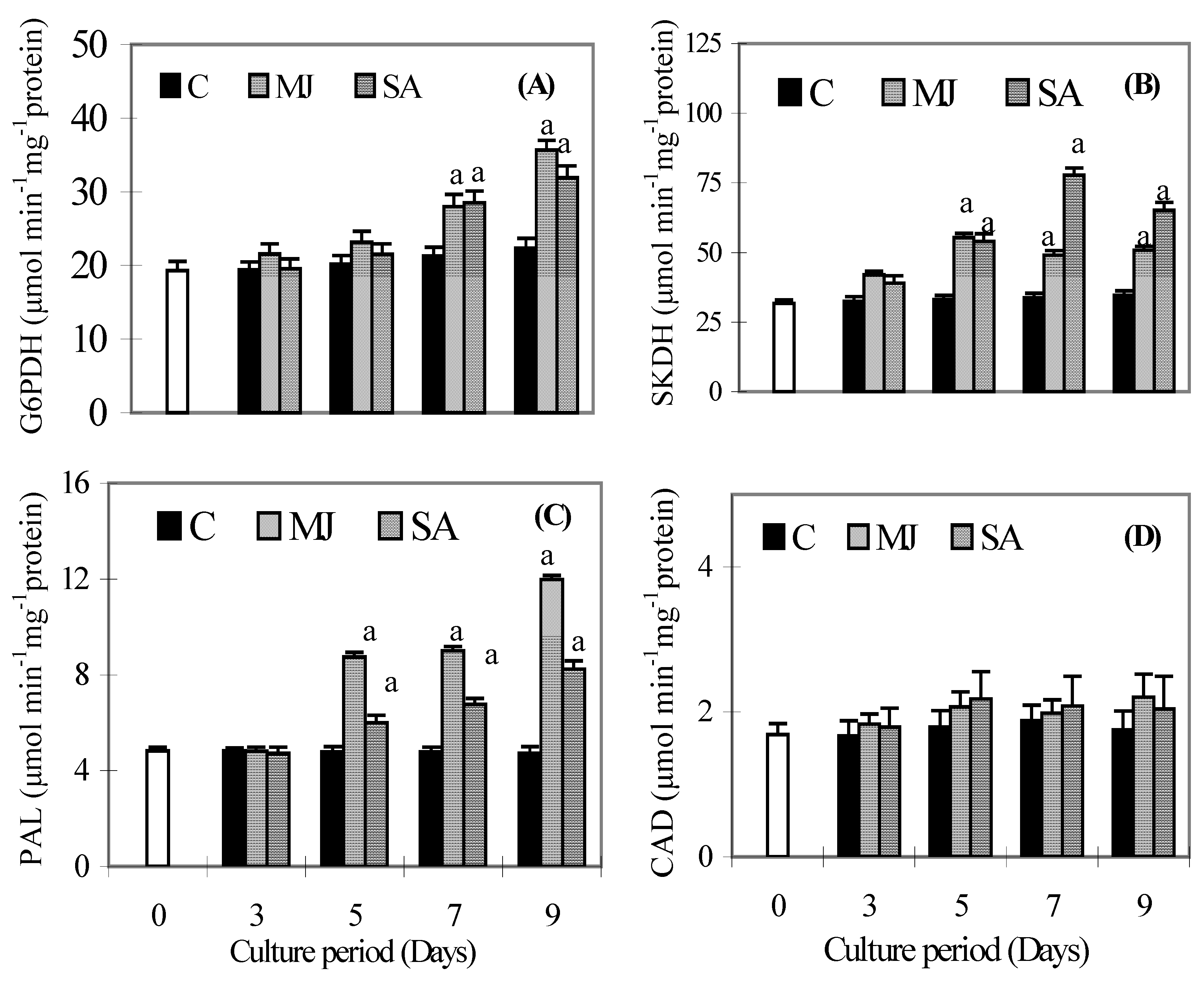
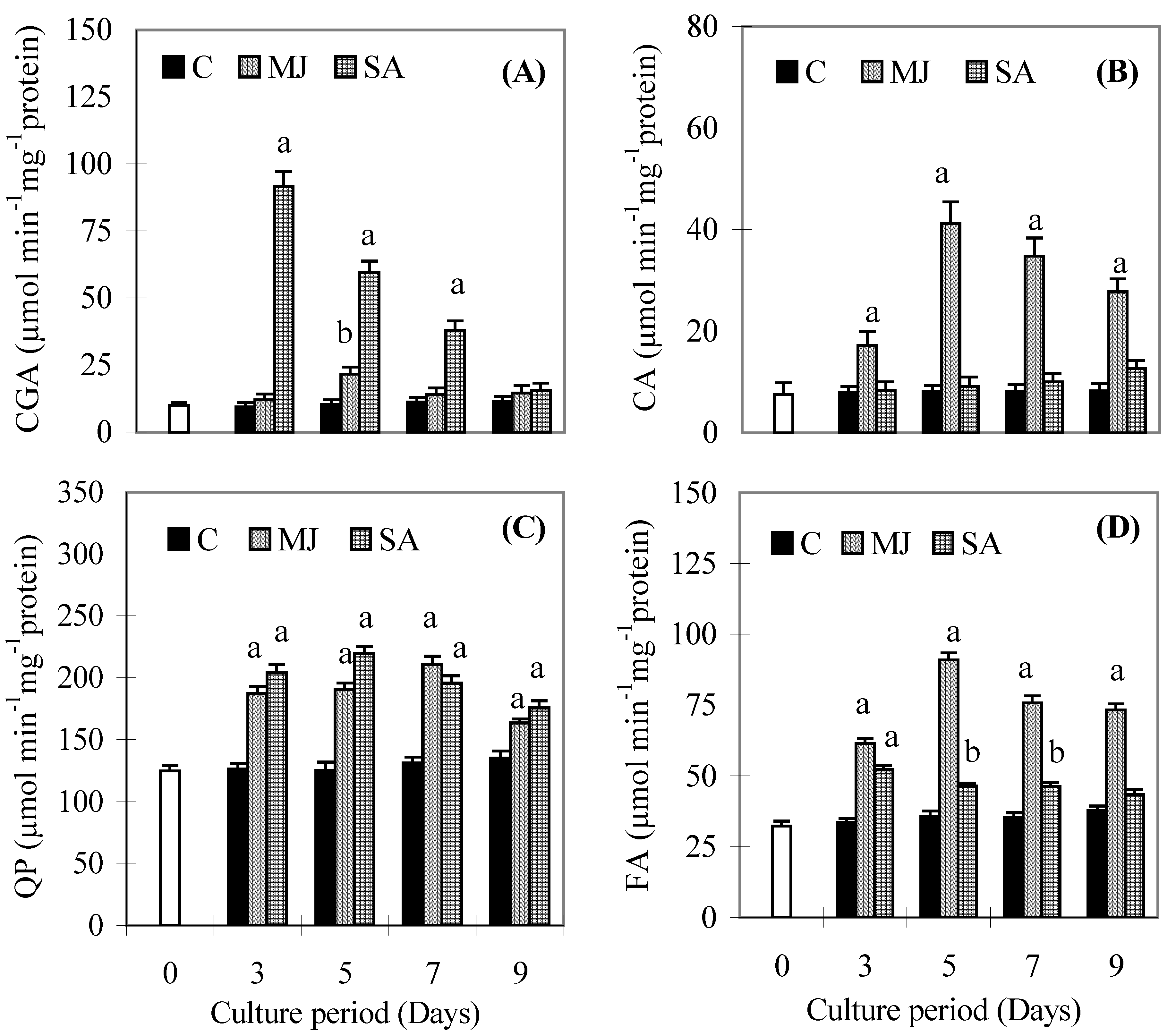
Discussion
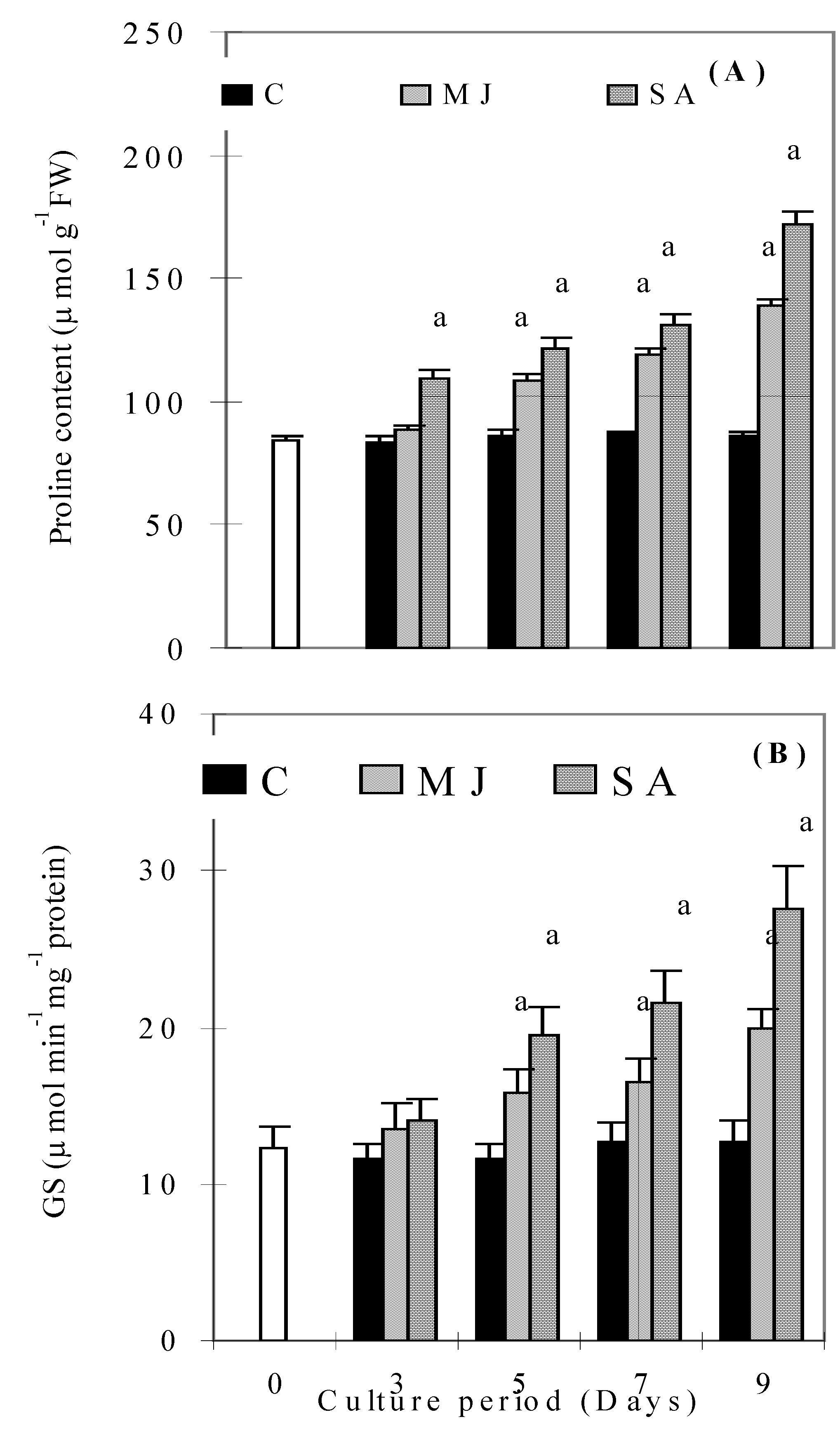
Conclusions
Experimental
Stock root cultures
Treatment with elicitors
Determination of root growth
Determination of protein carbonyl and hydrogen peroxide contents
Determination of total phenolics, flavonoids, ascorbic acid, cysteine and NPSH contents and DPPH activity
Determination of phenylpropanoid pathway enzymes
Determination of proline and β-GS activity
Statistics
Abbreviations
| CA | caffeic acid peroxidase |
| CGA | chlorogenic acid peroxidase |
| CAD | cinnamyl alcohol dehydrogenase |
| CBN-1 | Chungbuk National University line-1 |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| FA | ferulic acid peroxidase |
| G6PDH | glucose 6 phosphate dehydrogenase |
| β-GS | β-glucosidase |
| MJ | methyl jasmonate |
| NPSH | non-protein thiol |
| PAL | phenylalanine ammonia lyase |
| QP | quercetin peroxidase |
| SA | salicylic acid |
| SKDH | shikimate dehydrogenase |
Acknowledgments
References
- Solecka, D.; Kacperska, A. Phenylpropanoid deficiency affects the course of plant acclimation to cold. Physiol. Plant. 2003, 119, 253–262. [Google Scholar] [CrossRef]
- Ali, M.B.; Khatun, S.; Hahn, E.J.; Paek, K.Y. Enhancement of phenylpropanoid enzymes and lignin in Phalaenopsis orchid and their influence on plant acclimatisation at different levels of photosynthetic photon flux. Plant Grow Regul. 2006, 49, 137–146. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Weaver, L.M.; Herrmann, K.M. Dynamics of the shikimate pathway in plants. Trends Plant Sci. 1997, 2, 346–351. [Google Scholar]
- Randhir, R.; Vattem, D.A.; Shetty, K. Antioxidant enzyme response studies in H2O2-streesed procine muscle tissue following treatment with faba bean sprout extract and L-DOPA. J. Food Biochem. 2006, 30, 671–698. [Google Scholar] [CrossRef]
- Ratledge, C. Nutrition, growth and metabolism. In The Biology of the Mycobacteria; Ratledge, C., Stanford, J.L., Eds.; Academic Press: London, 1982; vol. 1, pp. 185–27. [Google Scholar]
- Blanco, P.R.; Medina, E.N.; Lopez, R.J.A.; Gonzalez, R.J.A.; Villalba, J.M.; Moyano, E.; Caballero, J.L.; Munoz, B.J. Cloning, expression and immunolocalization pattern of a cinnamyl alcohol dehydrogenase from strawberry. J. Exp. Bot. 2002, 53, 723–1734. [Google Scholar]
- Cvikrová, M.; Malá, J.; Hrubcová, M.; Eder, J. Soluble and cell wall-bound phenolics and lignin in Ascocalyx abietina infected Norway spruces. Plant Sci. 2006, 170, 563–570. [Google Scholar] [CrossRef]
- Schlag, E.M.; McIntosh, M.S. Ginsenoside content and variation among and within American ginseng (Panax quinquefolius L.) populations. Phytochemistry 2006, 67, 1510–1519. [Google Scholar]
- Gillis, C.N. Panax ginseng pharmacology: A nitric oxide link? Biochemical Pharmacol. 1997, 54, 1–8. [Google Scholar] [CrossRef]
- Ding, C.K.; Wang, C.Y.; Gross, K.C.; Smith, D.L. Jasmonate and salicylate induce the expression of pathogenesis-related-proteins genes and increase resistance to chilling injury in tomato fruit. Planta 2002, 214, 895–901. [Google Scholar] [CrossRef]
- Lee, A.; Cho, K.; Jang, S.; Rakwal, R.; Iwahashi, H.; Agrawal, G.K.; Shim, J.; Han, O. Inverse correlation between jasmonic acid and salicylic acid during early wound response in rice. Biochem. Biophys. Res. Commun. 2004, 318, 734–738. [Google Scholar]
- Ali, M.B.; Yu, K.W.; Hahn, E.J.; Paek, K.Y. Methyl jasmonate and salicylic acid elicitation induces ginsenosides accumulation, enzymatic and non-enzymatic antioxidant in suspension culture Panax ginseng roots in bioreactors. Plant Cell Rep. 2006, 25, 613–620. [Google Scholar]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism and nutritional significance. Nut. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Conrath, U.; Pieterse, C.M.J.; Mauch-Mani, B. Priming in plant–pathogen interactions. Trends Plant Sci. 2002, 7, 210–216. [Google Scholar] [CrossRef]
- Tan, J.; Schneider, B.; Svatos, A.; Bednarek, P.; Liu, J.; Hahlbrock, K. Universally occurring phenylpropanoid and species-specific indolic metabolites in infected and uninfected Arabidopsis thaliana roots and leaves. Phytochemistry 2004, 65, 691–699. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.R.; Mira, L. Protection by flavonoids against the peroxynitrite-mediated oxidation of dihydrorhodamine. Free Radic. Res. 2004, 38, 1011–1018. [Google Scholar] [CrossRef]
- Kim, H.J.; Chen, F.; Wang, Xi; Choi, J.H. Effect of methyl jasmonate on phenolics, isothiocyanate, and metabolic enzymes in radish sprout (Raphanus sativus L.). J. Agric. Food Chem. 2006, 54, 7263–7269. [Google Scholar]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar]
- Smith, I.K.; Vierheller, J.L.; Thorne, C.A. Properties and function of glutathione reductase in plants. Physiol. Plant. 1989, 77, 449–456. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Jyothsnakumari, G.; Sudhakar, C. Effects of jasmonic acid on groundnut during early seedling growth. Biol. Plant. 2003/2004, 47, 453–456. [Google Scholar]
- Misra, N.; Gupta, A.K. Effect of salt stress on proline metabolism in two high yielding genotypes of green gram. Plant Sci. 2005, 169, 331–339. [Google Scholar]
- Esen, A. β-glucosidases: overview. In β-glucosidases: biochemistry and molecular biology; Esen, A., Ed.; American Chemical Society: Washington DC, 1993; pp. 1–14. [Google Scholar]
- Mazzuca, S.; Uccella, N. β-glucosidase releasing of phytoalexin derivatives from secobiophenols as defence mechanism against pathogenic elicitors in olive drupes. Acta Hort. 2002, 586, 529–531. [Google Scholar]
- Reymond, P.; Farmer, E.E. Jasmonate and salicylate as global signals for defense gene expression. Curr. Opin. Plant Biol. 1998, 1, 404–411. [Google Scholar] [CrossRef]
- Thulke, O.; Conrath, U. Salicylic acid has a dual role in the activation of defence-related genes in parsley. Plant J. 1998, 14, 35–42. [Google Scholar]
- Brader, J.L.G.; Palva, E.T. The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate mediated signals in plant defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef]
- Rao, M.V.; Lee, H.I.; Creelman, R.A.; Mullet, J.E.; Davis, K.R. Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 2000, 12, 1633–1646. [Google Scholar]
- Farrell, R. L.; Murtagh, K. E.; Tien, M.; Mozuc, M. D.; Kirk, T. K. Physical and enzymatic properties of lignin-peroxidase isoenzymes from Phanerochaete chrysosporium. Enz. Microb. Technol. 1989, 11, 322–328. [Google Scholar]
- Kobayashi, A.; Koguchi, Y.; Kanzaki, H.; Kajiyama, S.; Kawazu, K. A new type of antimicrobial phenolics produced by plant peroxidase and its possible role in the chemical defense system against plant pathogens. Z. Naturforsch. 1994, 49, 411–414. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue. Physiol. Plant. 1962, 15, 473–497. [Google Scholar]
- Levine, R.; Tenhaken, R.; Dixon, R.A.; Lamb, C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994, 79, 583–593. [Google Scholar]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Shetty, K.K.; Curtis, O.F.; Levin, RE.; Withowsky, R.; Ang, W. Prevention of vitrification associated with in vitro shoot cultures of oregano (Origanum vulgare) by Pseudomona spp. J. Plant Physiol. 1995, 147, 447–451. [Google Scholar] [CrossRef]
- Zhuang, X.; Lu, P.Y.Y.; Yang, G.S. Extraction and determination of flavonoid in ginkgo. Chin. Herb. Med. 1992, 23, 122–124. [Google Scholar]
- Franke, W. Ascorbinsaure. In Moderne Methoden der Pflanzenanalyse; Paech, K., Tracey, M.V., Eds.; Springer: Berlin, 1955; Vol. 2, pp. 95–112. [Google Scholar]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice: their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gaitonde, M.K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 1967, 104, 627–633. [Google Scholar]
- Ellman, G.L. Tissue sulfhydril groups. Arch. Biochem. Biophys. 1959, 82, 70–71. [Google Scholar]
- Debnam, P.M.; Emes, M.J. Subcellular distribution of enzymes of the oxidative pentose phosphate pathway in root and leaf tissues. J. Exp. Bot. 1999, 50, 1653–61. [Google Scholar]
- Díaz, J.; Ros, Barceló, A.; Merino, F. Changes in shikimate dehydrogenase and the end products of the shikimate pathway, chlorogenic acid and lignins, during the early development of seedlings of Capsicum annuum. New Phytol. 1997, 136, 183–88. [Google Scholar]
- Mitchell, H.J.; Hall, J.L.; Barber, M.S. Elicitor induced cinnamyl alcohol dehydrogenase activity in lignifying wheat (Triticum aestivum L.) leaves. Plant Physiol. 1994, 104, 551–56. [Google Scholar]
- Raag, H.; Kuhn, D.N.; Kahlbroeck, K. Coordinated regulation of coumarate: CoA ligase and phenylalanine ammonia lyase mRNA in cultured plant cells. J. Biol. Chem. 1984, 256, 52–60. [Google Scholar]
- Mäder, M.; Nessel, A.; Boff, M. On the physiological significance of the isozyme groups of peroxidase from tobacco demonstrated by biochemical properties. Z. Pflanzenphysiol. 1977, 82, 247–60. [Google Scholar] [CrossRef]
- Francisco, J.P.; Daniel, V.; Nilo, M. Ascorbic acid and flavonoid-peroxidase reaction as a detoxifying system of H2O2 in grapevine leaves. Phytochemistry 2002, 60, 573–580. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.K. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–208. [Google Scholar] [CrossRef]
- Legin, E.; Copinet, A.; Duchiron, F. Production of thermostable amylolytic enzymes by Thermococcus hydrothermalis. Biotechnol Letts. 1998, 20, 363–367. [Google Scholar] [CrossRef]
- Sample Availability: Contact the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Ali, M.B.; Hahn, E.-J.; Paek, K.-Y. Methyl Jasmonate and Salicylic Acid Induced Oxidative Stress and Accumulation of Phenolics in Panax ginseng Bioreactor Root Suspension Cultures. Molecules 2007, 12, 607-621. https://doi.org/10.3390/12030607
Ali MB, Hahn E-J, Paek K-Y. Methyl Jasmonate and Salicylic Acid Induced Oxidative Stress and Accumulation of Phenolics in Panax ginseng Bioreactor Root Suspension Cultures. Molecules. 2007; 12(3):607-621. https://doi.org/10.3390/12030607
Chicago/Turabian StyleAli, Mohammad Babar, Eun-Joo Hahn, and Kee-Yoeup Paek. 2007. "Methyl Jasmonate and Salicylic Acid Induced Oxidative Stress and Accumulation of Phenolics in Panax ginseng Bioreactor Root Suspension Cultures" Molecules 12, no. 3: 607-621. https://doi.org/10.3390/12030607




