Synthesis of α,β-Unsaturated Ketones as Chalcone Analogues via a SRN1 Mechanism
Abstract
:Introduction
Results and Discussion
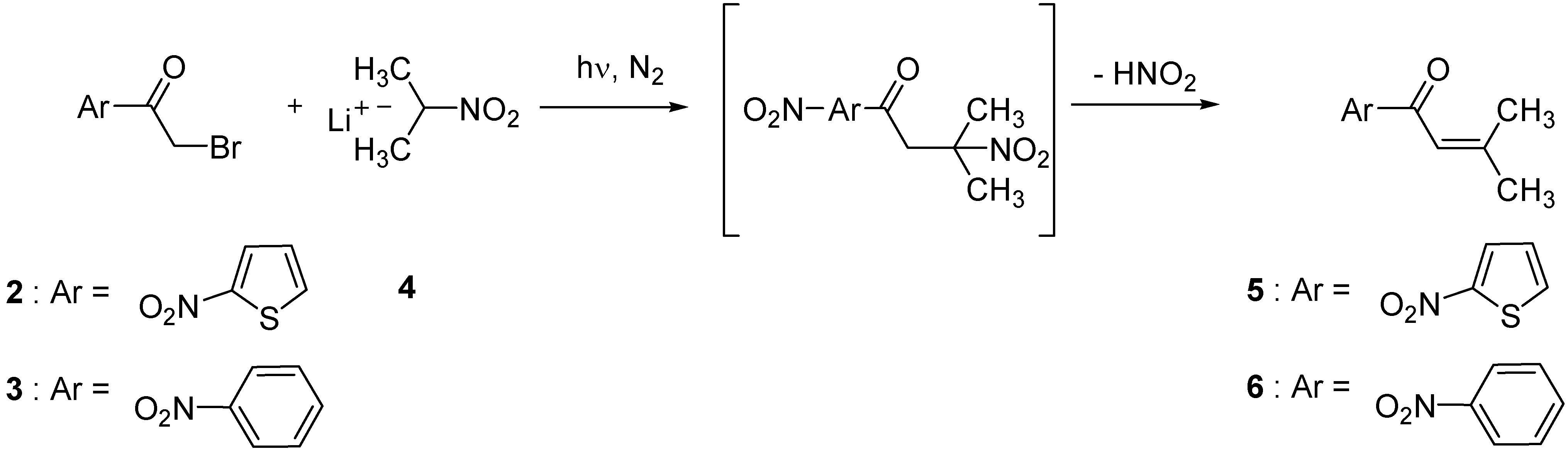
| Substrate | 2-nitropropyl anion equivalents | Solvent | Product obtained | Yield (%)b |
|---|---|---|---|---|
| 2 | 2 | DMF | 5 | 20 |
| 2 | 2 | DMSO | 5 | 18 |
| 2 | 2 | MeOH | 5 | 90 |
| 3 | 1 | DMF | 6 | 42 |
| 3 | 2 | DMF | 6 | 45 |
| 3 | 3 | DMF | 6 | 48 |
| 3 | 2 | DMSO | 6 | 35 |
| 3 | 2 | MeOH | 6 | 56 |
| 3 | 3 | MeOH | 6 | 63 |
| Inhibitor | Yield in nitrothiophene series, 5 (%)b | Yield in nitrobenzene series, 6 (%)b |
|---|---|---|
| - | 90 | 63 |
| O2 | 65 | 31 |
| Dark | 72 | 37 |
| O2 + Dark | 58 | 18 |
| TEMPO (0.1 eq.) | 21 | 17 |
| TEMPO (1 eq.) | 13 | 9 |
| p-NO2C6H4NO2 (0.1 eq.) | 19 | 15 |
| p-NO2C6H4NO2 (1 eq.) | 8 | 7 |
| CuCl2, 2H2O (0.1 eq.) | 5 | 5 |
| Substrate | Anion | Product | Yield (%)b |
|---|---|---|---|
| 2 |  |  | 85 |
| 2 |  |  | 70 |
| 3 |  |  | 45 |
| 3 |  |  | 48 |
| 3 |  |  | 39 |
Experimental
General
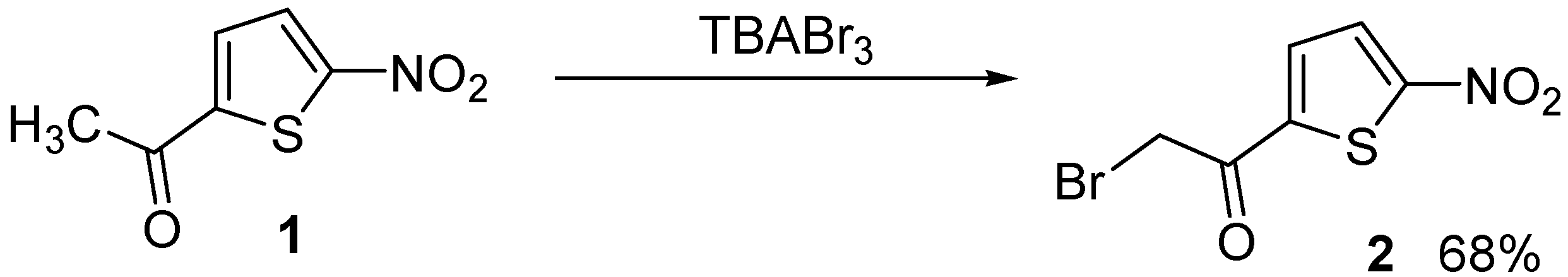
Preparation of 2-bromo-1-(5-nitrothiophen-2-yl)ethanone using tetrabutylammonium tribromide
Reaction of α-bromoketones with 2-nitropropyl anion
Mechanistic study of the reaction between α-bromoketones and 2-nitropropyl anion
Generalization of SRN1 reaction of α-bromoketones with other nitronate anions.
Acknowledgments
References
- Marzinzik, A.L.; Felder, E.R. Key Intermediates in Combinatorial Chemistry: Access to Various Heterocycles from α,β-unsaturated ketones on Solid Phase. J. Org. Chem. 1998, 63, 723–727. [Google Scholar]
- Srikanth, G.S.C.; Castle, S.L. Advances in radical conjugate additions. Tetrahedron 2005, 61, 10377–10441. [Google Scholar]
- Furusawa, M.; Tanaka, T.; Ito, T.; Nishiwaka, A.; Yamazaki, N.; Nakaya, K.I.; Matsuura, N.; Tsuchiya, H.; Nagayama, M.; Iinuma, M. Antioxidant Activity of Hydroxyflavonoids. J. Health Sci. 2005, 51, 376–378. [Google Scholar] [CrossRef]
- Dimmock, J.R.; Jha, A.; Zello, G.A.; Quail, J.W.; Oloo, E.O.; Nienaber, K.H.; Kowalczyk, E.S.; Allen, T.M.; Santos, C.L.; De Clerck, E.; Balzarini, J.; Manavathu, E.K.; Stables, J.P. Cytotoxic N-[4-(3-aryl-3-oxo-1-propenyl)phenylcarbonyl]-3,5-bis(phenylmethylene)-4-piperi-dones and related compounds. Eur. J. Med. Chem. 2002, 37, 961–972. [Google Scholar]
- Nam, N.H.; Hong, D.H.; You, Y.J.; Kim, Y.; Bang, S.C.; Kim, H.M.; Ahn, B.Z. Synthesis and cytotoxicity of 2,5-dihydroxychalcones and related compounds. Arch. Pharm. Res. 2004, 27, 581–588. [Google Scholar]
- Yayli, N.; Goek, Y.; Uecuencue, O.; Yasar, A.; Atasoy, C.; Sahinbas, E.; Kuecuek, M. Stereoselective photochemistry of substituted chalcones in solution and their antioxidant activities. J. Chem. Res. 2005, 3, 155–159. [Google Scholar] [CrossRef]
- Bardia, R.; Rao, J.T. Synthesis and antimicrobial activity of some α,β-unsaturated aromatic ketones. Asian J. Chem. 2004, 16, 1194–1196. [Google Scholar]
- Remusat, V.; Terme, T.; Gellis, A.; Rathelot, P.; Vanelle, P. Synthesis of original benzo[g]quinoxaline-5,10-diones by bis-SRN1 methodology. J. Heterocycl. Chem. 2004, 41, 221–225. [Google Scholar] [CrossRef]
- Njoya, Y.; Gellis, A.; Crozet, M.P.; Vanelle, P. Efficient synthesis of new 6-nitrobenzothiazoles using microwave irradiation. Sulfur Lett. 2003, 26, 67–75. [Google Scholar]
- Vanelle, P.; Gellis, A.; Kaafarani, M.; Maldonado, J.; Crozet, M.P. Fast electron transfer C-alkylation of 2-nitropropane anion under microwave irradiation. Tetrahedron Lett. 1999, 40, 4343–4346. [Google Scholar]
- Russell, G.A.; Ros, F. Electron transfer processes. 34. Reactions of α-haloketones with Nucleophiles. J. Am. Chem. Soc. 1985, 107, 2506–2411. [Google Scholar]
- Kajigaeshi, S.; Kakinami, T.; Okamoto, T.; Fujisaki, S. Synthesis of bromoacetyl derivatives by use of tetrabutylammonium tribromide. Bull. Chem. Soc. Jpn. 1987, 60, 1159–1160. [Google Scholar]
- Chanon, M.; Tobe, M.L. ETC: A Mechanistic Concept for Inorganic and Organic Chemistry. Angew. Chem., Int. Ed. Engl. 1982, 21, 1–23. [Google Scholar]
- Vanelle, P. (Ed.) Electron Transfer Reactions in Organic Synthesis; Research Signpost: Trivandrum, 2002; and references therein.
- Deady, L.W.; Stanborough, M.S. The synthesis of 2-(heteroaryl)imidazo[1,2-a]pyridin-3-ols and related compounds. Aust. J. Chem. 1981, 34, 1295–1302. [Google Scholar]
- Sample Availability: Not available.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Curti, C.; Gellis, A.; Vanelle, P. Synthesis of α,β-Unsaturated Ketones as Chalcone Analogues via a SRN1 Mechanism. Molecules 2007, 12, 797-804. https://doi.org/10.3390/12040797
Curti C, Gellis A, Vanelle P. Synthesis of α,β-Unsaturated Ketones as Chalcone Analogues via a SRN1 Mechanism. Molecules. 2007; 12(4):797-804. https://doi.org/10.3390/12040797
Chicago/Turabian StyleCurti, Christophe, Armand Gellis, and Patrice Vanelle. 2007. "Synthesis of α,β-Unsaturated Ketones as Chalcone Analogues via a SRN1 Mechanism" Molecules 12, no. 4: 797-804. https://doi.org/10.3390/12040797




