How Many Drugs Are Catecholics
Abstract
:Introduction
Results and Discussion
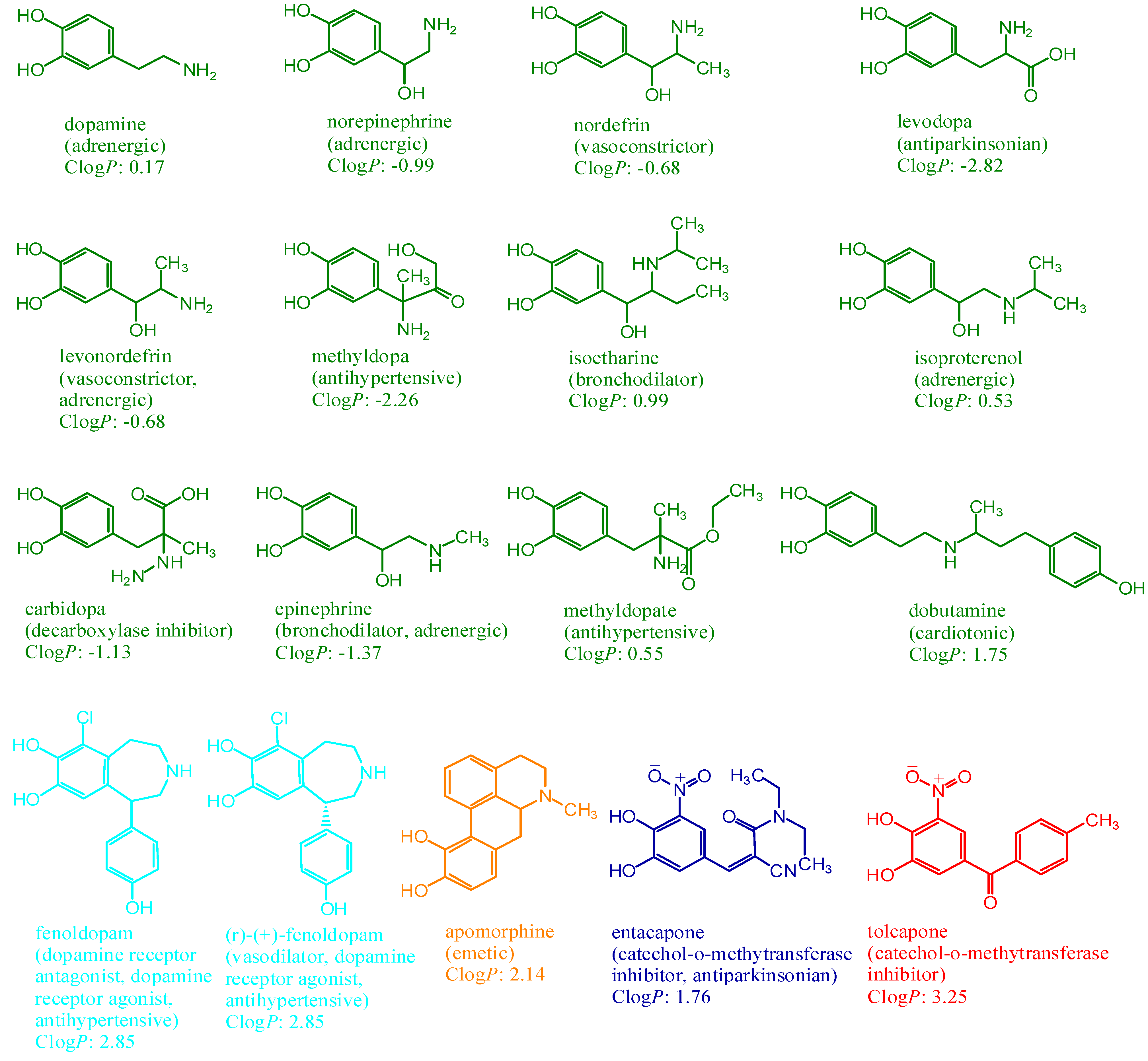

Conclusions
Acknowledgments
Supplementary Material
References and Notes
- Shahidi, F. (Ed.) Natural Antioxidants: Chemistry, Health Effects, and Applications; The American Oil Chemistss Society: Champaign, IL, 1997.
- Thomas, C. E. Handbook of Synthetic Antioxidants; Packer, L., Cadenas, E., Eds.; Marcel Dekker, Inc.: New York, 1997; pp. 1–52. [Google Scholar]
- Zhang, H.-Y. Structure-activity relationships and rational design strategies for radical- scavenging antioxidants. Curr. Comput.-Aided Drug Des. 2005, 1, 257–273. [Google Scholar] [CrossRef]
- Zhang, H.-Y. Theoretical investigation on free radical scavenging activity of 6,7-dihydroxyflavone. Quant. Struct. -Act. Relat. 2000, 19, 50–53. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Sun, Y.-M.; Wang, X.-L. Substituent effects on O-H bond dissociation enthalpies and ionization potentials of catechols: a DFT study and its implications in rational design of phenolic antioxidants and elucidation of structure-activity relationships for flavonoid antioxidants. Chem. Eur. J. 2003, 9, 502–508. [Google Scholar] [CrossRef]
- Foti, M. C.; Johnson, E. R.; Vinqvist, M. R.; Wright, J. S.; Barclay, L. R. C.; Ingold, K. U. Naphthalene Diols: A new class of antioxidants intramolecular hydrogen bonding in catechols, naphthalene diols, and their aryloxyl radicals. J. Org. Chem. 2002, 67, 5190–5196. [Google Scholar]
- Hussain, H. H.; Babic, G..; Durst, T.; Wright, J. S.; Flueraru, M.; Chichirau, A.; Chepelev, L. L. Development of novel antioxidants: design, synthesis, and reactivity. J. Org. Chem. 2003, 68, 7023–7032. [Google Scholar] [CrossRef]
- Chichirau, A.; Flueraru, M.; Chepelev, L. L.; Wright, J. S.; Willmore, W. G.; Durst, T.; Hussain, H. H.; Charron, M. Mechanism of cytotoxicity of catechols and a naphthalenediol in PC12-AC cells: the connection between extracellular autoxidation and molecular electronic structure. Free Radic. Biol. Med. 2005, 38, 344–355. [Google Scholar] [CrossRef]
- Flueraru, M.; Chichirau, A.; Chepelev, L. L.; Willmore, W. G.; Durst, T.; Charron, M.; Barclay, L. R. C.; Wright, J. S. Cytotoxicity and cytoprotective activity in naphthalenediols depends on their tendency to form naphthoquinones. Free Radic. Biol. Med. 2005, 39, 1368–1377. [Google Scholar] [CrossRef]
- Bolton, J. L.; Trush, M. A.; Penning, T. M.; Dryhurst, G.; Monks, T. J. Role of quinones in toxicology. Chem. Res. Toxicol. 2000, 13, 135–160. [Google Scholar]
- Comprehensive Medicinal Chemistry, Release 2004.1; MDL Information Systems Inc.: San Leandro, CA, 2004.
- Cytotoxicity and cytoprotective activity of naphthalenediols in rat cortical neurons. Chem. Res. Toxicol. 2006, 19, 1221–1227. [CrossRef]
- The Hammett constants σm and σp of NO2 are 0.71 and 0.78, respectively: Hantsch, C.; Leo, A.; Taft, R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [CrossRef]
- Verma, R. P.; Kapur, S.; Barberena, O.; Shusterman, A.; Hansch, C. H.; Selassie, C. D. Synthesis, cytotoxicity, and QSAR analysis of X-thiophenols in rapidly dividing cells. Chem. Res. Toxicol. 2003, 16, 276–284. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A. J. Substituent Constants for Correlation Analysis in Chemistry and Biology; Wiley: New York, NY, 1979. [Google Scholar]
- CambridgeSoft, Cambridge, MA, USA. 2006.
- Moridani, M. Y.; Siraki, A.; O’Brien, P. J. Quantitative structure toxicity relationships for phenols in isolated rat hepatocytes. Chem.-Biol. Interact. 2003, 145, 213–223. [Google Scholar] [CrossRef]
- Although this method is not very accurate for calculating catecholic O-H BDEs [3], it is suitable to help draw qualitative conclusions
- Dewar, M. J. S.; Zoebisch, E. G.; Healy, E. F.; Stewart, J. J. P. Development and use of quantum mechanical molecular models. 76. AM1: a new general purpose quantum mechanical molecular model. J. Am. Chem. Soc. 1985, 107, 3902–3909. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A. D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar]
- Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Zakrzewski, V. G.; Montgomery, J. A.; Stratmann, R. E.; Burant, J. C.; Dapprich, S.; Millam, J. M.; Daniels, A. D.; Kudin, K. N.; Strain, M. C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G. A.; Ayala, P. Y.; Cui, Q.; Morokuma, K.; Salvador, P.; Dannenberg, J. J.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Cioslowski, J.; Ortiz, J. V.; Baboul, A. G.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaroni, I.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Andres, J. L.; Gonzalez, C.; Head-Gordon, M.; Replogle, E. S.; Pople, J. A. Gaussian 98, Revision A.11; Gaussian, Inc.: Pittsburgh, PA, 2001. [Google Scholar]
- Morphy, R.; Kay, C.; Rankovic, Z. From magic bullets to designed multiple ligands. Drug Discov. Today. 2004, 9, 641–651. [Google Scholar] [CrossRef]
- Mencher, S. K.; Wang, L. G. Promiscuous drugs compared to selective drugs (promiscuity can be a virtue). BMC Clin. Pharmacol. 2005, 5, 3. [Google Scholar]
- Morphy, R.; Rankovic, Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef]
- Keith, C. T.; Borisy, A. A.; Stockwell, B. R. Multicomponent therapeutics for networked systems. Nat. Rev. Drug Discov. 2005, 4, 71–78. [Google Scholar] [CrossRef]
- Hopkins, A. L.; Mason, J. S.; Overington, J. P. Can we rationally design promiscuous drugs? Curr. Opin. Struct. Biol. 2006, 16, 127–136. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Yang, D.-P.; Tang, G.-Y. Multifunctional antioxidants: from screening to design. Drug Discov. Today 2006, 11, 749–754. [Google Scholar]
- Zhang, H.-Y. One-compound-multiple-targets strategy to combat Alzheimer’s disease. FEBS Lett. 2005, 579, 5260–5264. [Google Scholar]
- Zhang, H.-Y.; Yang, D.-P.; Ji, H.-F. Naturally occurring multipotent anti-Alzheimer agents. Expert Opin. Drug Discov. 2006, 1, 269–277. [Google Scholar] [CrossRef]
- Zhang, H.-Y. Same causes, same cures. Biochem. Biophys. Res. Commun. 2006, 351, 578–581. [Google Scholar] [CrossRef]
- It is noticeable that five of the 78 catecholic drugs, i.e., baicalein, fenoldopam, gossypol, oleuropein, (r)-(+)-fenoldopam, possess three or more pharmacological effects (Supplementary Information - Table S1)
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Yang, D.-P.; Ji, H.-F.; Tang, G.-Y.; Ren, W.; Zhang, H.-Y. How Many Drugs Are Catecholics. Molecules 2007, 12, 878-884. https://doi.org/10.3390/12040878
Yang D-P, Ji H-F, Tang G-Y, Ren W, Zhang H-Y. How Many Drugs Are Catecholics. Molecules. 2007; 12(4):878-884. https://doi.org/10.3390/12040878
Chicago/Turabian StyleYang, Da-Peng, Hong-Fang Ji, Guang-Yan Tang, Wei Ren, and Hong-Yu Zhang. 2007. "How Many Drugs Are Catecholics" Molecules 12, no. 4: 878-884. https://doi.org/10.3390/12040878
APA StyleYang, D. -P., Ji, H. -F., Tang, G. -Y., Ren, W., & Zhang, H. -Y. (2007). How Many Drugs Are Catecholics. Molecules, 12(4), 878-884. https://doi.org/10.3390/12040878



