β-Orcinol Metabolites from the Lichen Hypotrachyna revoluta
Abstract
:Introduction
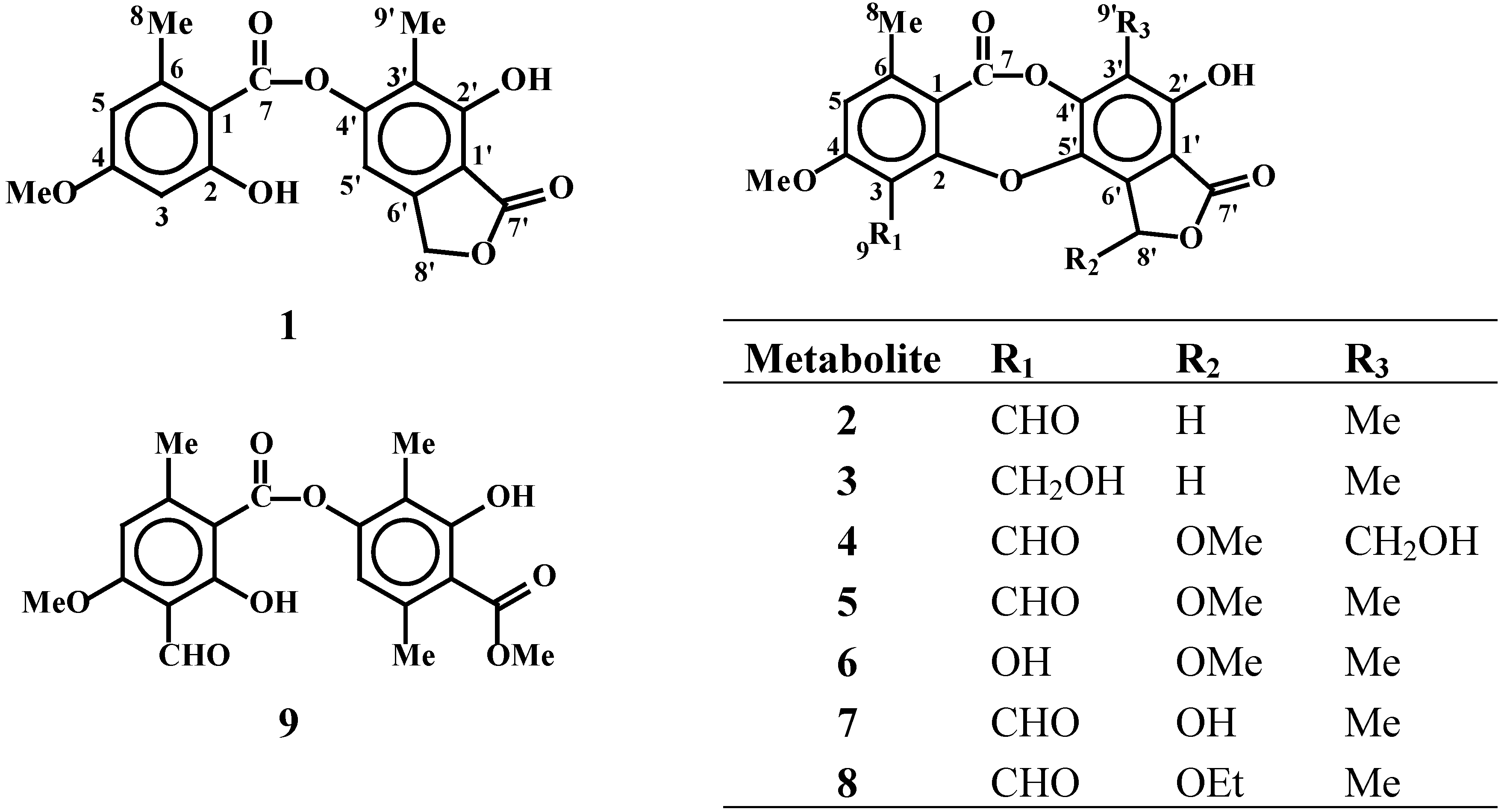
Results and Discussion
| 1 | 2 | 3 | 4a | 5 | ||||||
| δC | δH | δC | δH | δC | δH | δC | δH | δC | δH | |
| 1 | 114.5 | 114.4 | 113.7 | 113.9 | ||||||
| 2 | 163.2 | 161.3 | 159.3 | 160.1 | ||||||
| 3 | 100.9 | 6.73 (brs) | 113.6 | 121.5 | 114.6 | |||||
| 4 | 161.6 | 163.33 | 160.8 | 163.6 | ||||||
| 4-OCH3 | 56.0 | 3.82 (s) | 56.6 | 3.96 (s) | 56.2 | 3.91 (s) | 56.7 | 3.98 (s) | 56.5 | 3.95 (s) |
| 5 | 111.3 | 6.58 (brs) | 111.7 | 6.74 (s) | 112.6 | 6.65 (s) | 112.4 | 6.81 (s) | 112.0 | 6.73 (s) |
| 6 | 144.9 | 151.1 | 144.7 | 151.7 | ||||||
| 7 | 169.3 | nd | nd | nd | ||||||
| 8 | 22.6 | 2.44 (s) | 22.4 | 2.56 (s) | 21.5 | 2.51 (s) | 22.2 | 2.55 (s) | 22.1 | 2.54 (s) |
| 9 | 187.2 | 10.45 (s) | 53.6 | 4.79 (s) | 186.9 | 10.47 (s) | 187.3 | 10.47 (s) | ||
| 1’ | 107.2 | 106.9 | 104.8 | 107.2 | ||||||
| 2’ | 152.9 | 152.2 | 151.8 | 152.0 | ||||||
| 3’ | 120.9 | 117.7 | 119.9 | 121.4 | ||||||
| 4’ | 150.1 | 148.9 | 149.4 | 149.3 | ||||||
| 5’ | 101.3 | 7.17 (s) | 134.0 | 131.6 | nd | |||||
| 6’ | nd | 137.1 | 134.3 | 132.2 | ||||||
| 7’ | 164.4 | 171.8 | 172.3 | 169.0 | ||||||
| 8’ | 58.6 | 5.31
(d, J=13.2) 5.17 (d, J=13.2) | 70.1 | 5.43 (s) | 67.1 | 5.64 (s) | 109.4 | 6.35 (s) | 102.3 | 6.38 (s) |
| 9’ | 8.9 | 2.20 (s) | 8.9 | 2.27 (s) | 9.0 | 2.27 (s) | 55.1 | 4.90 (s) | 9.1 | 2.27 (s) |
| 2’-OH | nd | 7.95 (brs) | 7.87 (brs) | nd | 7.87 (brs) | |||||
| 8’-OCH3 | 57.4 | 3.65 (s) | 57.5 | 3.68 (s) | ||||||
- nd = not detected
- a 13C-NMR assignments from indirect HMQC detection, due to insufficient sample quantities.
Radical scavenging activity
| Metabolite | Radical scavenging activity (expressed as Trolox® equivalents) # |
|---|---|
| 1 | 85.14 ± 0.60 |
| 2 | 13.76 ± 1.14 |
| 3 | not active |
| 5 | 61.85 ± 1.65 |
| 6 | 7.63 ± 0.68 |
| 7 | 104.34 ± 2.70 |
| 9 | 11.85 ± 0.64 |
Experimental
General
Plant material
Extraction and Isolation
Hydroxyl radical - scavenging activity (CL)
Acknowledgments
References
- Vartia, K.O. The Lichens; Ahmadjian, V., Hale, M.E., Eds.; Academic Press: New York, 1974; p. 547. [Google Scholar]
- Rundel, P.W. The Ecological Roleof Secondary Lichen Substances. Biochem. Syst. Ecol. 1978, 6, 157–170. [Google Scholar] [CrossRef]
- Ingólfsdóttir, K.; Chung, G.A.C.; Skúlason, V.G.; Gissurarson, S.R.; Vilhelmsdóttir, M. Antimycobacterial activity of lichen metabolites in vitro. Eur. J. Pharm. Sci. 1998, 6, 141–144. [Google Scholar]
- Perry, N.B.; Benn, M.H.; Brennan, N.J.; Burgess, E.J.; Ellis, G.; Galloway, D.J.; Lorimer, S.D.; Tangney, R.S. Antimicrobial, antiviral and cytotoxic activity of New Zealand lichens. Lichenologist 1999, 31, 627–636. [Google Scholar] [CrossRef]
- Richardson, D.H.S. Handbook of Lichenology; Calun, M., Ed.; CRC Press: Boca Raton, 1988; Vol. 3, p. 93. [Google Scholar]
- Richardson, D.H.S. Frontiers in Mycology; Hawksworth, D.L., Ed.; CAB International: Wallingford, 1991; p. 187. [Google Scholar]
- Huneck, S.; Yoshimura, I. Identification of Lichen Substances; Springer-Verlag: Berlin, 1996. [Google Scholar]
- Hidalgo, M.E.; Fernandez, E.; Quilhot, W.; Lissi, E. Antioxidant Activity of Depsides and Depsidones. Phytochemistry 1994, 37, 1585–1587. [Google Scholar] [CrossRef]
- Culberson, C.F. Chemical and Botanical Guide to Lichen Products; The University of North Carolina Press: Chapel -Hill, NC, 1969. [Google Scholar]
- Nahas, R.; Abatis, D.; Anagnostopoulou, M. A.; Kefalas, P.; Vagias, C.; Roussis, V. Radical scavenging activity of Aegean sea marine algae. Food Chem. 2007, 102, 577–581. [Google Scholar] [CrossRef]
- Guri, A.; Kefalas, P.; Roussis, V. Antioxidant potential of six pine species. Phytother. Res. 2006, 20, 263–266. [Google Scholar]
- Tziveleka, L.-A.; Kourounakis, A.P.; Kourounakis, P.N.; Roussis, V.; Vagias, C. Antioxidant potential of natural and synthesized polyprenylated hydroquinones. Bioorg. Med. Chem. 2002, 10, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Kathirgamanathar, S.; Williams, D.E.; Andersen, R.J.; Bombuwela, K.; De Silava, D.; Karunaratne, V. β-Orcinol depsidones from the lichen Usnea sp. from Sri Lanka. Nat. Prod. Res. 2005, 19, 695–701. [Google Scholar] [CrossRef]
- Shimada, S.; Saitoh, T.; Sankawa, U.; Shibata, S. New depsidones from Lobaria oregano. Phytochemistry 1980, 19, 328–330. [Google Scholar] [CrossRef]
- González, A.G.; Barrera, J.B.; Perez, E.M.R.; Padron, C.E.H. Depsidones from Lobaria pulmonaria and their chemotaxonomic importance. Biochem. Syst. Ecol. 1994, 22, 583–586. [Google Scholar]
- Huneck, S.; Tabacchi, R. ψ-Esters of depsidones with a lactole ring. Phytochemistry 1987, 26, 1131–1138. [Google Scholar] [CrossRef]
- Walker, J.R.L.; Lintott, E.A. A phytochemical register of New Zealand lichens. New Zeal. J. Bot. 1997, 35, 369–384. [Google Scholar]
- Parejo, I.; Codina, C.; Petrakis, C.; Kefalas, P. Evaluation of scavenging activity assessed by Co(II)/EDTA-induced luminol chemiluminescence and DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical assay. J. Pharmacol. Toxicol. Methods 2000, 44, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Contact the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Papadopoulou, P.; Tzakou, O.; Vagias, C.; Kefalas, P.; Roussis, V. β-Orcinol Metabolites from the Lichen Hypotrachyna revoluta. Molecules 2007, 12, 997-1005. https://doi.org/10.3390/12050997
Papadopoulou P, Tzakou O, Vagias C, Kefalas P, Roussis V. β-Orcinol Metabolites from the Lichen Hypotrachyna revoluta. Molecules. 2007; 12(5):997-1005. https://doi.org/10.3390/12050997
Chicago/Turabian StylePapadopoulou, Panagiota, Olga Tzakou, Constantinos Vagias, Panagiotis Kefalas, and Vassilios Roussis. 2007. "β-Orcinol Metabolites from the Lichen Hypotrachyna revoluta" Molecules 12, no. 5: 997-1005. https://doi.org/10.3390/12050997





