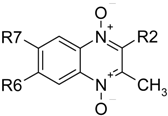
Substitutions of Fluorine Atoms and Phenoxy Groups in the Synthesis of Quinoxaline 1,4-di-N-oxide Derivatives
Abstract
:Introduction
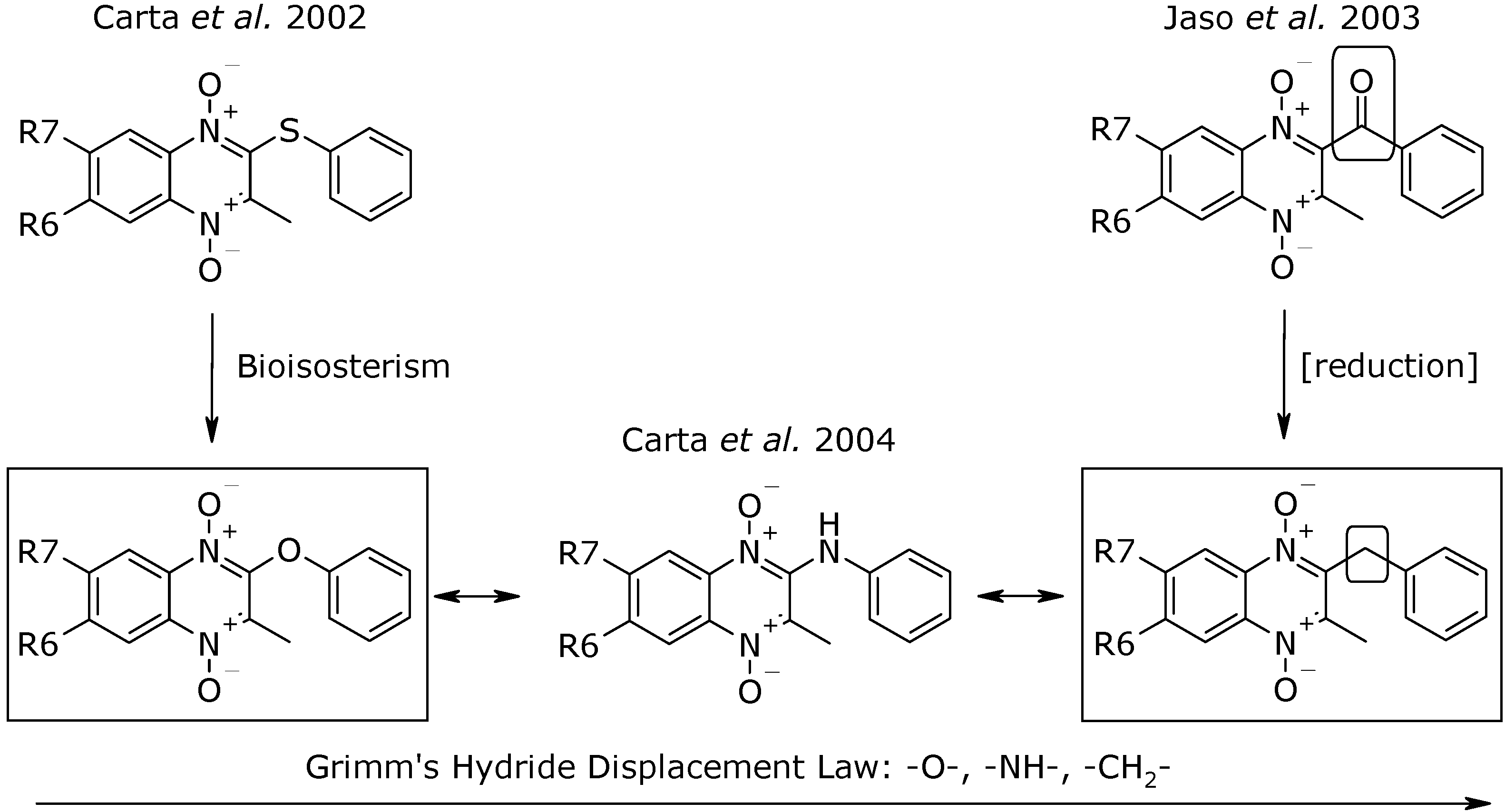
Results and Discussion
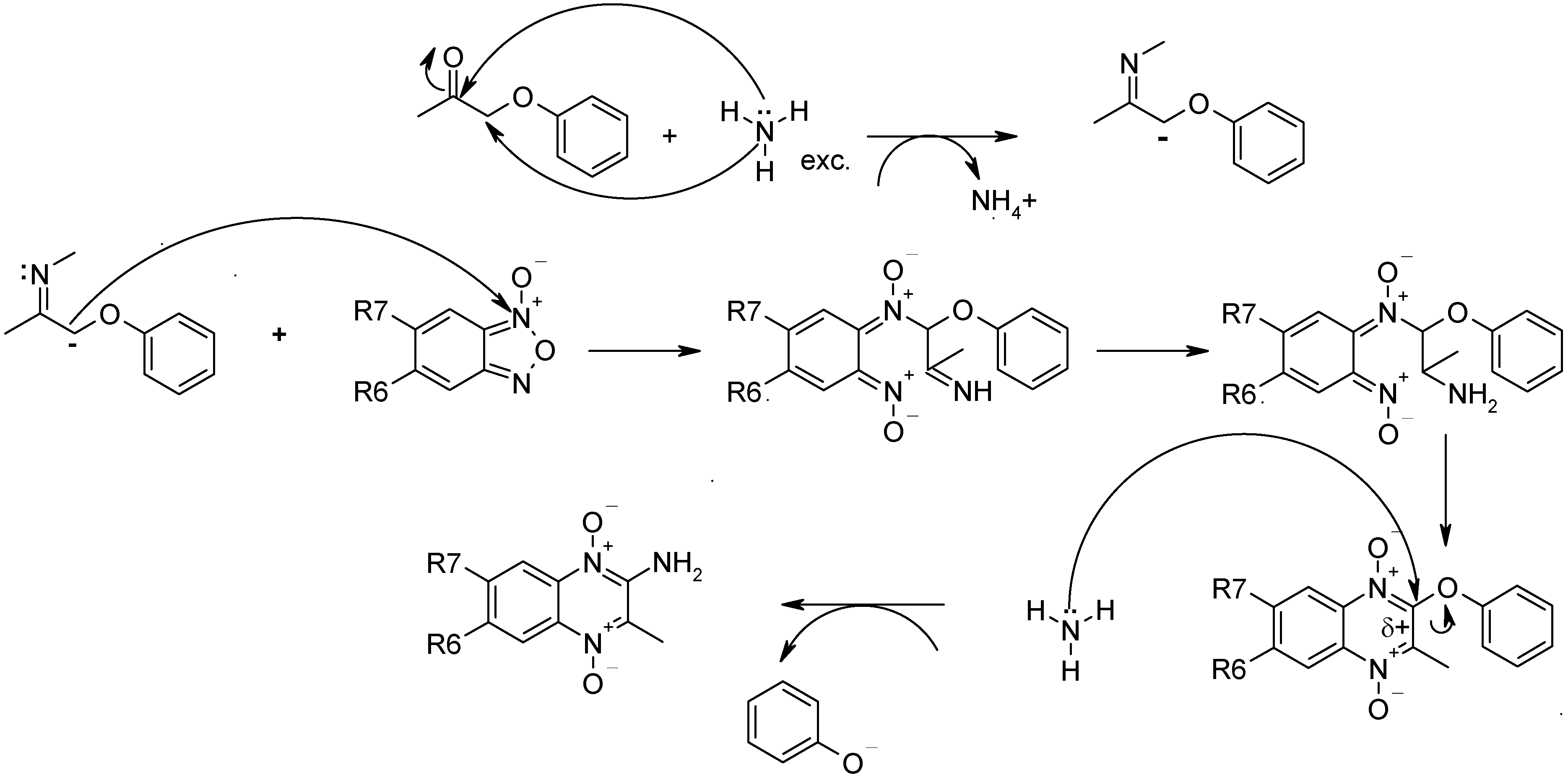
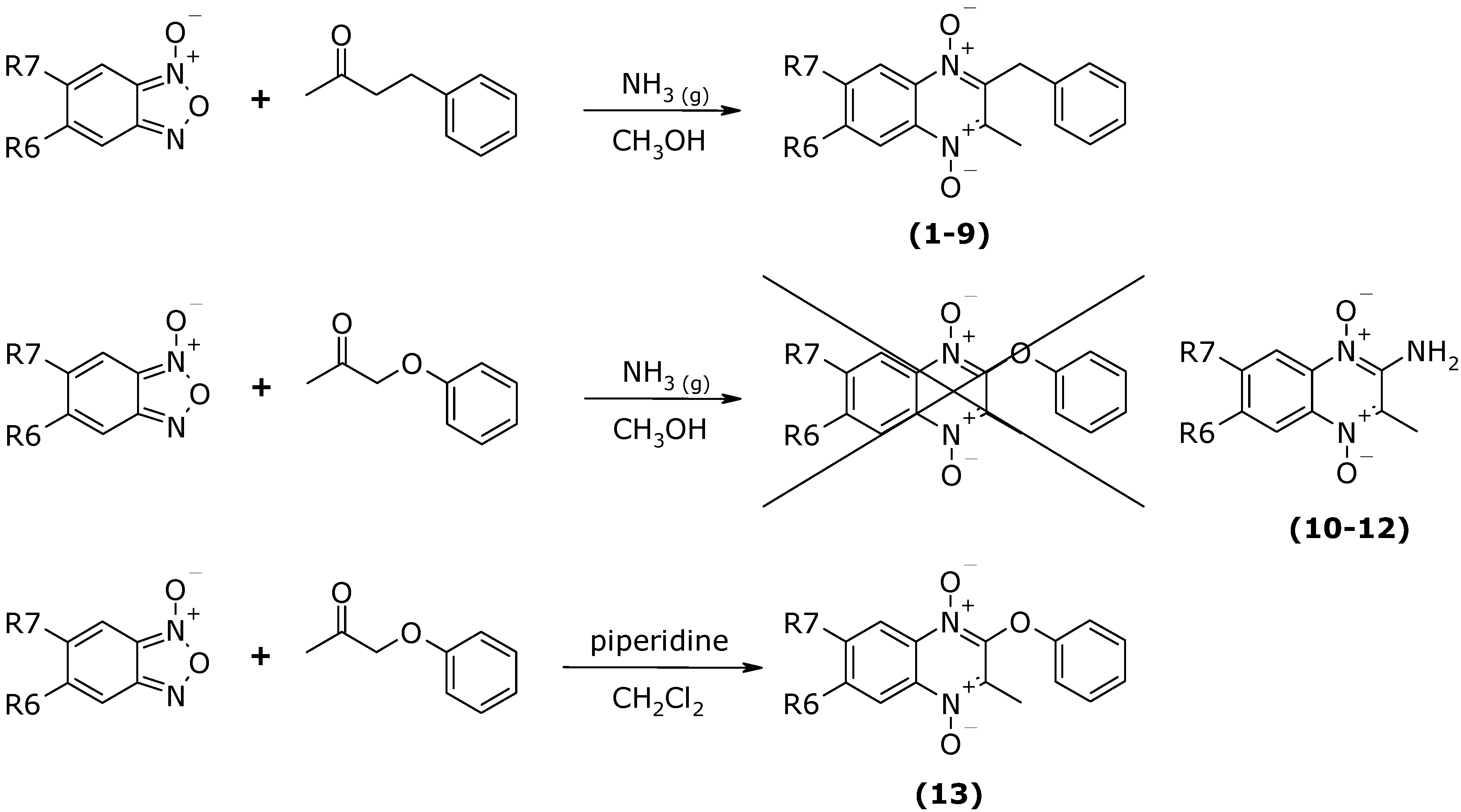
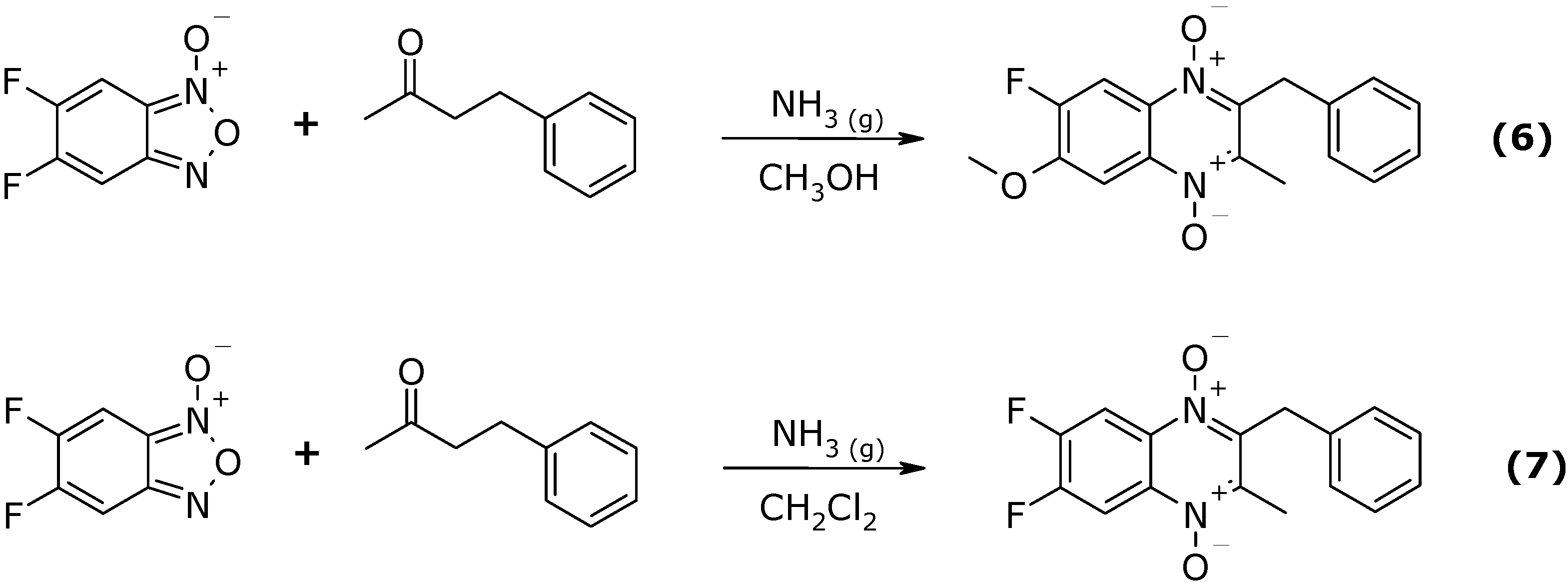
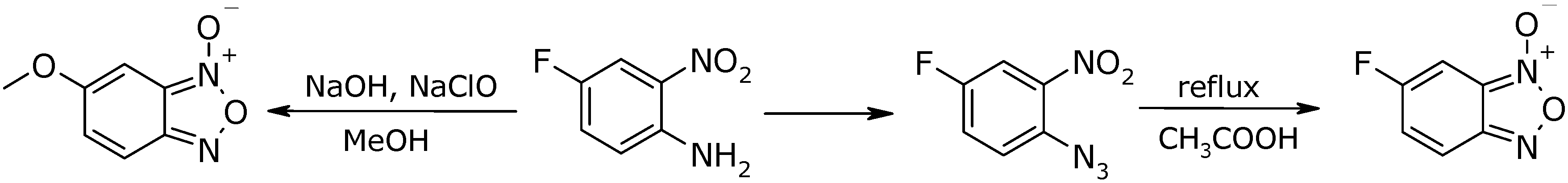
Conclusions
Experimental
General
General synthesis of 2-benzyl-3-methylquinoxaline 1,4-di-N-oxide derivatives 1-9 [11, 19]
General synthesis of 2-amino-3-methylquinoxaline 1,4-di-N-oxide derivatives 10-12.
Synthesis of 3-methyl-2-phenoxyquinoxaline 1,4-di-N-oxide (13).
| ID | R2 | R6 | R7 | Formula | MW | Calc. C (found) | Calc. H (found) | Calc. N (found) | MS (EI, 70 eV): m/z |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CH2-Ph | H | H | C16H14N2O2 | 266.30 | 72.17 (71.78) | 5.30 (5.27) | 10.52 (10.24) | 266 ([M·]+), 249, 232 |
| 2 | CH2-Ph | H | F | C16H13FN2O2 | 284.29 | 67.60 (67.34) | 4.61 (4.52) | 9.85 (10.19) | 284 ([M·]+), 267, 250 |
| 3 | CH2-Ph | H | Cl | C16H13ClN2O2 | 300.75 | 63.90 (64.01) | 4.36 (4.30) | 9.31 (9.42) | 300 ([M·]+), 283, 266 |
| 4 | CH2-Ph | H | CH3 | C17H16N2O2 | 280.33 | 72.84 (72.45) | 5.75 (5.66) | 9.99 (10.30) | 280 ([M·]+), 263, 246, 230 |
| 5 | CH2-Ph | H | OCH3 | C17H16N2O3 | 296.33 | 68.91 (68.59) | 5.44 (5.34) | 9.45 (9.58) | 296 ([M·]+), 279, 262, 247, 219 |
| 6 | CH2-Ph | F | OCH3 | C17H15FN2O3 | 314.32 | 64.96 (65.01) | 4.81 (4.83) | 8.91 (8.69) | 314 ([M·]+), 297, 280, 265, 237 |
| 7 | CH2-Ph | F | F | C16H12F2N2O2 | 302.28 | 63.58 (63.61) | 4.00 (3.70) | 9.27 (9.00) | 302 ([M·]+), 285, 268 |
| 8 | CH2-Ph | Cl | Cl | C16H12Cl2N2O4 | 335.19 | 57.33 (57.04) | 3.61 (3.52) | 8.36 (7.99) | 334 ([M·]+), 317, 300, 285 |
| 9 | CH2-Ph | CH3 | CH3 | C18H18N2O2 | 294.36 | 73.45 (73.16) | 6.16 (6.16) | 9.52 (9.41) | 294 ([M·]+), 277, 260, 245 |
| 10 | NH2 | H | H | C9H9N3O2·H2O | 191.19+18.02 | 51.67 (51.61) | 5.30 (5.52) | 20.09 (19.77) | 191 ([M·]+), 173, 145 |
| 11 | NH2 | H | Cl | C9H8ClN3O2·½H2O | 225.64+9.01 | 46.03 (46.40) | 3.83 (3.63) | 17.90 (17.51) | 225 ([M·]+), 208, 191 |
| 12 | NH2 | Cl | Cl | C9H7Cl2N3O2 | 260.08 | 41.56 (41.90) | 2.71 (2.72) | 16.16 (15.78) | 259 ([M·]+), 243, 226 |
| 13 | O-Ph | H | H | C15H12N2O3 | 268.27 | 67.16 (66.81) | 4.51 (4.52) | 10.44 (10.05) | 268 ([M·]+), 251, 234, 175 |
Acknowledgements
References and Notes
- Sakata, G.; Makino, K.; Kurasawa, Y. Recent Progress in the Quinoxaline Chemistry - Synthesis and Biological-Activity. Heterocycles 1988, 27, 2481–2515. [Google Scholar]
- Carta, A.; Corona, P.; Loriga, M. Quinoxaline 1,4-dioxide: A versatile scaffold endowed with manifold activities. Curr. Med. Chem. 2005, 12, 2259–2272. [Google Scholar] [CrossRef]
- Carta, A.; Piras, S.; Loriga, G.; Paglietti, G. Chemistry, biological properties and SAR analysis of quinoxalinones. Mini-Rev. Med. Chem. 2006, 6, 1179–1200. [Google Scholar]
- Li, X.; Yang, K. H.; Li, W. L.; Xu, W. F. Recent advances in the research of quinoxalinone derivatives. Drugs Future 2006, 31, 979–989. [Google Scholar]
- Lima, L. M.; Barreiro, E. J. Bioisosterism: A useful strategy for molecular modification and drug design. Curr. Med. Chem. 2005, 12, 23–49. [Google Scholar] [CrossRef]
- Inbaraj, J. J.; Motten, A. G.; Chignell, C. F. Photochemical and photobiological studies of tirapazamine (SR 4233) and related quinoxaline 1,4-di-N-oxide analogues. Chem. Res. Toxicol. 2003, 16, 164–170. [Google Scholar] [CrossRef]
- Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Synthesis of new 2-acetyl and 2-benzoyl quinoxaline 1,4-di-N-oxide derivatives as anti-Mycobacterium tuberculosis agents. Eur. J. Med. Chem. 2003, 38, 791–800. [Google Scholar] [CrossRef]
- Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A. Synthesis and antimycobacterial activity of new quinoxaline-2-carboxamide 1,4-di-N-oxide derivatives. Bioorg. Med. Chem. 2003, 11, 2149–2156. [Google Scholar] [CrossRef]
- Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Synthesis of new quinoxaline-2-carboxylate 1,4-dioxide derivatives as anti-Mycobacterium tuberculosis agents. J. Med. Chem. 2005, 48, 2019–2025. [Google Scholar]
- Aldana, I.; Ortega, M. A.; Jaso, A.; Zarranz, B.; Oporto, P.; Giménez, A.; Monge, A.; Deharo, E. Anti-malarial activity of some 7-chloro-2-quinoxalinecarbonitrile-1,4-di-N-oxide derivatives. Pharmazie 2003, 58, 68–69. [Google Scholar]
- Aguirre, G.; Cerecetto, H.; Di Maio, R.; Gonzalez, M.; Alfaro, M. E. M.; Jaso, A.; Zarranz, B.; Ortega, M. A.; Aldana, I.; Monge-Vega, A. Quinoxaline N,N'-dioxide derivatives and related compounds as growth inhibitors of Trypanosoma cruzi. Structure-activity relationships. Bioorg. Med. Chem. Lett. 2004, 14, 3835–3839. [Google Scholar]
- Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A.; Maurel, S.; Deharo, E.; Jullian, V.; Sauvain, M. Synthesis and antimalarial activity of new 3-arylquinoxaline-2-carbonitrile derivatives. Arzneim.-Forsch. 2005, 55, 754–761. [Google Scholar]
- Zarranz, B.; Jaso, A.; Lima, L. M.; Aldana, I.; Monge, A.; Maurel, S.; Sauvain, M. Antiplasmodial activity of 3-trifluoromethyl-2-carbonylquinoxaline di-N-oxide derivatives. Braz. J. Pharm. Sci. 2006, 42, 357–361. [Google Scholar]
- Marín, A.; Lima, L. M.; Solano, B.; Vicente, E.; Pérez-Silanes, S.; Maurel, S.; Sauvain, M.; Aldana, I.; Monge, A.; Deharo, E. Antiplasmodial structure-activity relationship of 3-trifluoromethyl-2-arylcarbonylquinoxaline 1,4-di-N-oxide derivatives. Exp. Parasitol. 2008, 118, 25–31. [Google Scholar] [CrossRef]
- Vicente, E.; Charnaud, S.; Bongard, E.; Villar, R.; Burguete, A.; Solano, B.; Ancizu, S.; Pérez-Silanes, S.; Aldana, I.; Vivas, L.; Monge, A. Synthesis and antiplasmodial activity of 3-furyl and 3-thienylquinoxaline-2-carbonitrile 1,4-di-N-oxide derivatives. Molecules 2008, 13, 69–77. [Google Scholar] [CrossRef]
- Vicente, E.; Lima, L. M.; Bongard, E.; Charnaud, S.; Villar, R.; Solano, B.; Burguete, A.; Pérez-Silanes, S.; Aldana, I.; Vivas, L.; Monge, A. Synthesis and structure-activity relationship of 3-phenylquinoxaline 1,4-di-N-oxide derivatives as antimalarial agents. Eur. J. Med. Chem. 2008. In Press. [Google Scholar] [CrossRef]
- Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A. Synthesis and anticancer activity evaluation of new 2-alkylcarbonyl and 2-benzoyl-3-trifluoromethyl-quinoxaline 1,4-di-N-oxide derivatives. Bioorg. Med. Chem. 2004, 12, 3711–3721. [Google Scholar] [CrossRef]
- Solano, B.; Junnotula, V.; Marín, A.; Villar, R.; Burguete, A.; Vicente, E.; Pérez-Silanes, S.; Aldana, I.; Monge, A.; Dutta, S.; Sarkar, U.; and Gates, K. S. Synthesis and biological evaluation of new 2-arylcarbonyl-3-trifluoromethylquinoxaline 1,4-di-N-oxide derivatives and their reduced analogs. J. Med. Chem. 2007, 50, 5485–5492. [Google Scholar] [CrossRef]
- Carta, A.; Loriga, M.; Paglietti, G.; Mattana, A.; Fiori, P. L.; Mollicotti, P.; Sechi, L.; Zanetti, S. Synthesis, anti-mycobacterial, anti-trichomonas and anti-candida in vitro activities of 2-substituted-6,7-difluoro-3-methylquinoxaline 1,4-dioxides. Eur. J. Med. Chem. 2004, 39, 195–203. [Google Scholar] [CrossRef]
- Cheeseman, G. W. H.; Cookson, R. F. The chemistry of heterocyclic compounds: a series of monographs; Wiley & Sons: New York, 1979. [Google Scholar]
- Ortega, M. A.; Morancho, M. J.; Martinez-Crespo, F. J.; Sainz, Y.; Montoya, M. E.; de Cerain, A. L.; Monge, A. New quinoxalinecarbonitrile 1,4-di-N-oxide derivatives as hypoxic-cytotoxic agents. Eur. J. Med. Chem. 2000, 35, 21–30. [Google Scholar] [CrossRef]
- Kotovskaya, S. K.; Romanova, S. A.; Charushin, V. N.; Kodess, M. I.; Chupakhin, O. N. 5(6)-fluoro-6(5)-R-benzofuroxans: synthesis and NMR 1H, 13C and 19F studies. J. Fluor. Chem. 2004, 125, 421–428. [Google Scholar] [CrossRef]
- Leyva, S.; Castanedo, V.; Leyva, E. Synthesis of novel fluorobenzofuroxans by oxidation of anilines and thermal cyclization of arylazides. J. Fluor. Chem. 2003, 121, 171–175. [Google Scholar] [CrossRef]
- Monge, A.; Palop, J. A.; De Cerain, A. L.; Senador, V.; Martinez-Crespo, F. J.; Sainz, Y.; Narro, S.; Garcia, E.; De Miguel, C.; Gonzalez, M.; Hamilton, E.; Barker, A. J.; Clarke, E. D.; Greenhow, D. T. Hypoxia-selective agents derived from quinoxaline 1,4-di-N-oxides. J. Med. Chem. 1995, 38, 1786–1792. [Google Scholar] [CrossRef]
- Sample availability: Contact the authors
© 2008 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Vicente, E.; Villar, R.; Burguete, A.; Solano, B.; Ancizu, S.; Pérez-Silanes, S.; Aldana, I.; Monge, A. Substitutions of Fluorine Atoms and Phenoxy Groups in the Synthesis of Quinoxaline 1,4-di-N-oxide Derivatives. Molecules 2008, 13, 86-95. https://doi.org/10.3390/molecules13010086
Vicente E, Villar R, Burguete A, Solano B, Ancizu S, Pérez-Silanes S, Aldana I, Monge A. Substitutions of Fluorine Atoms and Phenoxy Groups in the Synthesis of Quinoxaline 1,4-di-N-oxide Derivatives. Molecules. 2008; 13(1):86-95. https://doi.org/10.3390/molecules13010086
Chicago/Turabian StyleVicente, Esther, Raquel Villar, Asunción Burguete, Beatriz Solano, Saioa Ancizu, Silvia Pérez-Silanes, Ignacio Aldana, and Antonio Monge. 2008. "Substitutions of Fluorine Atoms and Phenoxy Groups in the Synthesis of Quinoxaline 1,4-di-N-oxide Derivatives" Molecules 13, no. 1: 86-95. https://doi.org/10.3390/molecules13010086
APA StyleVicente, E., Villar, R., Burguete, A., Solano, B., Ancizu, S., Pérez-Silanes, S., Aldana, I., & Monge, A. (2008). Substitutions of Fluorine Atoms and Phenoxy Groups in the Synthesis of Quinoxaline 1,4-di-N-oxide Derivatives. Molecules, 13(1), 86-95. https://doi.org/10.3390/molecules13010086