Experimental
General
Melting points (uncorrected) were determined with a Büchi melting points apparatus. IR spectra: Bruker Vector 22. Mass spectra: Varian MAT 311 A. NMR spectra: Bruker DRX 500 operating at 500 MHz (
1H) and 125 MHz (
13C), respectively; all spectra were recorded in CDCl
3. TLC: silica gel 60 F 254 (Merck). Column chromatography: Machery-Nagel Kieselgel 60 and Merck Kieselgel 60, mesh size 0.04-0.063. Elemental analyses were carried out with a Perkin Elmer CHN Analysator 263 at the Institute of Pharmaceutical Chemistry, University of Düsseldorf. 2-Coumaranone (
5a), allyl bromide and 1,3-dimethyltetrahydro-2(1
H)pyrimidone (DMPU) were purchased from Sigma-Aldrich Chemie GmbH and purified by distillation. Diisopropylamine (purity >99.5 %) was also purchased from Sigma-Aldrich. α,α’-Dibromo-o-xylene (purity 96 %) was obtained from the same supplier. It was refluxed over calcium hydride and distilled under nitrogen. General remarks concerning the handling of moisture and air-sensitive compounds are given in ref. [
14].
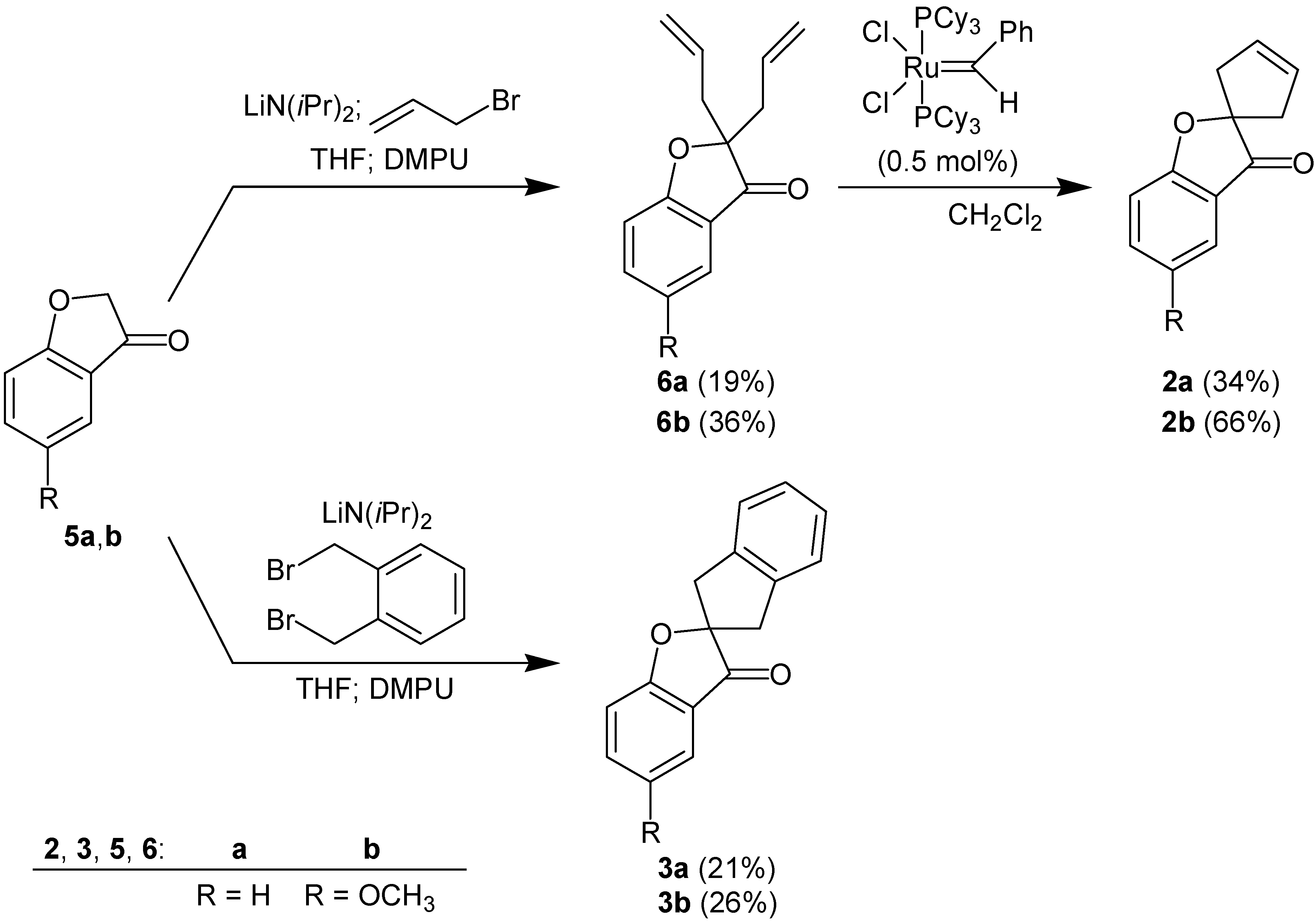
General procedure for the double allylation of benzofuranones 5 (G. P. 1):
A 250-mL Schlenk-flask was equipped with a magnetic stirrer, a thermocouple and a septum and was connected to a combined argon/vacuum line. The air in the flask was replaced by argon. Diisopropylamine (1.49 g, 2.07 mL, 14.7 mmol) and dry THF (52 mL) were injected, and the mixture was stirred at -78°C. A solution of n-butyllithium (9.75 mL, 15.6 mmol) in hexane was added at such a rate that the temperature did not exceed –70°C. Stirring was continued at 0°C for 30 min.
In a 50 mL Schlenk-flask, benzofuranone 5 (12.3 mmol) was dissolved in dry THF (20 mL) under argon. This solution was added through a cannula to the mixture of lithium diisopropylamide, prepared as described above, at –78°C. Stirring was continued at the same temperature for 2 h. Freshly distilled allyl bromide (1.73 g, 1.24 mL, 14.3 mmol) and 1,3-dimethyltetrahydro-2(1H)pyrimidone (DMPU) (1.89 g, 1.78 mL, 14.7 mmol) were injected. The mixture was allowed to warm up to room temperature overnight and stirred for 48 h at this temperature. The solution was washed with a saturated aqueous solution (50 mL) of NH4Cl. The aqueous solution was extracted with chloroform (3 times 50 mL). The organic layers were combined and dried with Na2SO4. The solvent was removed in a rotary evaporator and the oily residue was purified by column chromatography on silica gel. According to this procedure the following products were obtained:
2,2-Bis(prop-2-en-1-yl)-2,3-dihydro-1-benzofuran-3-one (6a): Prepared from 5a; Rf = 0.57 (n-hexane/ethyl acetate, 10 : 1); yield (relative to 5a): 19%; 1H-NMR: δ 2.53-2.63 (m, 4H, CH2CH=CH2), 5.02 (dd, J = 10.1 Hz, J = 1.6 Hz, 2H, CH2CH=CHH), 5.13 (dd, J = 17.3 Hz, J = 1.6 Hz, 2H, CH2CH=CHH), 5.59-5.67 (m, 2H, CH2CH=CH2), 7.03-7.26 (m, 2H, aromatic H), 7.58-7.60 (m, 2H, aromatic H); 13C-NMR: δ 39.9, 91.3, 113.2, 119.8, 121.4, 121.7, 124.2, 130.5, 138.1, 171.8, 203.2; IR (thin film, cm–1): 3050, 2955, 1710, 1490, 1435, 1340, 1275, 1245, 1215, 1140, 1030, 825, 755; MS (70 eV): m/z (%): 214 (28, [M]+), 199 (6), 185 (6), 172 (100), 147 (12), 145 (40), 128 (56), 115 (32), 91, (28), 76 (27), 65 (36), 55 (42); HRMS: (EI) calcd. for C14H14O2: 214.0994, found 214.0993.
5-Methoxy-2,2-bis(prop-2-en-1-yl)-2,3l-dihydro-1-benzofuran-3-one (6b): Prepared from 5b; Rf = 0.57 (ethyl acetate); yield (relative to 5b): 36%; 1H-NMR: δ 2.52-2.61 (m, 4H, CH2CH=CH2), 3.79 (s, 3H, OCH3), 5.02 (dd, J = 8.5 Hz, J = 1.0 Hz, 2H, CH2CH=CHH), 5.12 (dd, J = 17.0 Hz, J = 1.0 Hz, 2H, CH2CH=CHH), 5.58-5.63 (m, 2H, CH2CH=CH2), 7.00-7.10 (m, 2H, 4H, and 7-H), 7.24 (dd, J = 9.1 Hz, J = 2.8 Hz, 1H, 6-H); 13C-NMR: δ 40.0, 55.8, 92.0, 103.8, 114.1, 119.7, 121.2, 128.3, 130.6, 154.8, 167.2, 203.5; IR (thin film, cm–1): 3060, 2910, 1710, 1490, 1435, 1340, 1275, 1245, 1215, 1140, 1030, 825, 775; MS (70 eV): m/z (%): 244 (100, [M]+), 217 (13), 203 (88), 175 (21), 150 (32), 124 (12), 123 (19), 91 (15), 79 (18), 55 (27).
General procedure for the preparation of spiroannulated benzofuranones 2 by ring-closing metathesis (G. P. 2):
A mixture of benzylidene-bis-(tricyclohexylphosphino)-dichlororuthenium (“Grubbs catalyst I”) (0.016 g, 0.02 mmol) in dry dichloromethane (50 mL) was stirred under argon in a 100-mL flask equipped with a magnetic stirrer, a septum and a connection to the combined argon/vacuum line. At room temperature, diallyl compound 6 (4.0 mmol), dissolved in 6 mL of dry dichloromethane, was added and the mixture was stirred at room temperature for 19 h. The solvent was removed in a rotary evaporator and the residue was purified by column chromatography. According to this procedure the following products were obtained:
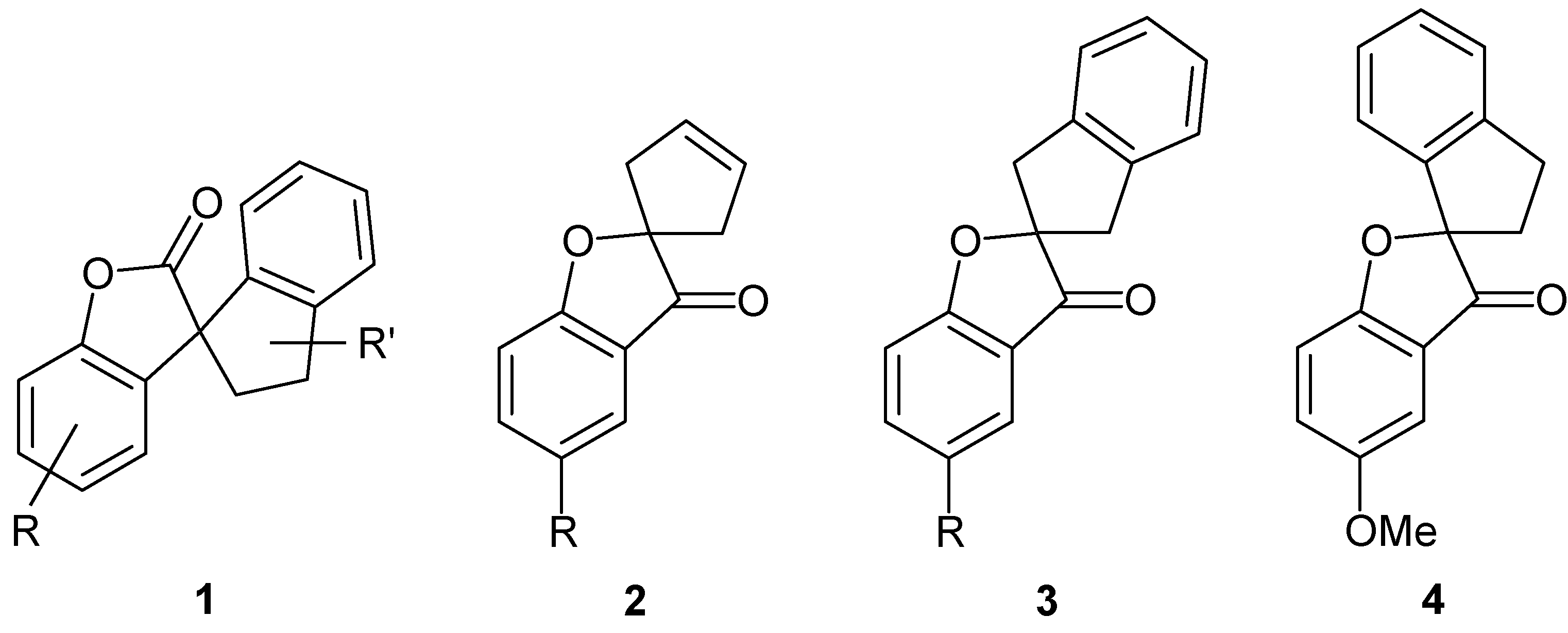
3H-Spiro[1-benzofuran-2,1’-cyclopentan]-3’-en-3-one (2a): Prepared from 6a; colorless oil; Rf = 0.43 (chloroform); yield: 34%; 1H-NMR: δ 2.69 (d, J = 17.0 Hz, 2H, 2’-H and 5’-H), 2.95 (d, J = 17.0 Hz, 2H, 2’-H and 5’-H), 5.81 (s, 2H, 3’-H and 4’-H), 7.07-7.10 (m, 2H, aromatic H), 7.60-7.64 (m, 2H, aromatic H); 13C-NMR: δ 44.1, 95.4, 113.4, 120.6, 212.8, 124.5, 127.8, 138.1, 171.4, 203.8; IR (thin film, cm–1): 3060, 2920, 2850, 1710, 1615, 1460, 1325, 1270, 1205, 1135, 1035, 945, 920, 845, 760, 680; MS (70 eV): m/z (%): 186 (100, [M]+), 171 (46), 157 (12), 121 (96), 92 (36). Anal. calcd. for C12O2H10: C, 77.40; H, 5.41. Found C, 77.71; H, 5.41.
5-Methoxy-3H-spiro[1-benzofuran-2,1’-cyclopentan]-3’-en-3-one (2b): Prepared from 6b; colorless oil; Rf = 0.71 (chloroform); yield: 66%; 1H-NMR: δ 2.68 (d, J = 16.8 Hz, 2H, 2’-H and 5’-H), 2.94 (d, J = 16.8 Hz, 2H, 2’-H and 5’-H), 3.80 (s, 3H, OCH3), 5.80 (s, 2H, 3’-H and 4’-H), 7.10 (d, J = 9.1 Hz, 1H, 7-H), 7.08 (d, J = 2.8 Hz, 1H, 4-H), 7.26 (dd, J = 9.1 Hz, J = 2.8 Hz, 1H, 6-H); 13C-NMR: δ 44.1, 55.9, 96.1, 104.1, 114.3, 120.3, 127.8, 128.2, 195.0, 166.9, 204.0; IR (thin film, cm–1): 3070, 2940, 2835, 1705, 1600, 1490, 1440, 1275, 1235, 1205, 1130, 1030, 875, 825, 755; MS (70 eV): m/z (%): 216 (61, [M]+), 204 (10), 151 (100), 150 (57).
General procedure for the preparation of spiroannulated benzofuranones 3 (G. P. 3):
According to G. P. 1, benzofuranones 5 were deprotonated with lithium diisopropylamide. Then, the solution was treated with 1 equivalent (relative to 5) of α,α’-dibromo-o-xylene, dissolved in THF, and 1 equivalent of DMPU. The mixture was warmed up to room temperature. After work up according to G. P. 1, the residue was purified by column chromatography. According to this procedure the following compounds were obtained:
1’,3’-Dihydro-3H-spiro[benzofuran-2,2’-indene]-3-one (3a): Prepared from 5a; colorless oil; Rf = 0.43 (n-hexane/ethyl acetate, 10:1); yield: 21%; 1H-NMR: δ 3.18 (d, J = 17.0 Hz, 2H, 1’-H and 3’-H), 3.55 (d, J = 17.0 Hz, 2H, 1’-H and 3’-H), 7.05-7.73 (m, 8H, aromatic H); 13C-NMR: δ 44.0, 97.2, 114.0, 121.0, 122.2, 125.0, 127.8, 138.7, 140.0, 171.8, 202.7; IR (thin film, cm–1): 2940, 1710, 1615, 1460, 1330, 1285, 1195, 1020, 915, 685; MS (70 eV): m/z (%): 236 (88, [M]+), 219 (11), 121 (100), 115 (41). Anal. calcd. for C17H12O2: C, 81.34; H, 5.12. Found C, 81.04; H, 5.15.
5-Methoxy-1’,3’dihydro-3H-spiro[1-benzofuran-2,2’-indene]-3-one (3b): Prepared from 5b; colorless oil; Rf = 0.73 (n-hexane/ethyl acetate, 2:1); yield: 26%; 1H-NMR: δ 3.18 (d, J = 16.7 Hz, 2H, 1’-H and 3’-H), 3.55 (d, J = 16.7 Hz, 2H, 1’-H and 3’-H), 3.82 (s, 3H, OCH3), 7.00 (d, J = 9.1 Hz, 1H, 7-H), 7.11 (d, J = 2.8 Hz, 1H, 4-H), 7.24-7.28 (m, 5H, remaining aromatic H); 13C-NMR: δ 43.7, 56.0, 97.5, 104.2, 114.5, 120.5, 124.6, 127.3, 128.4, 139.7, 155.0, 166.8, 202.5; IR (thin film, cm–1): 2940, 1700, 1490, 1350, 1280, 1230, 1195, 1125, 1025, 840, 740; MS (70 eV): m/z (%): 266 (19, [M]+), 207 (22), 205 (96), 183 (59), 140 (54), 105 (100), 103 (31), 78 (37), 58 (31).
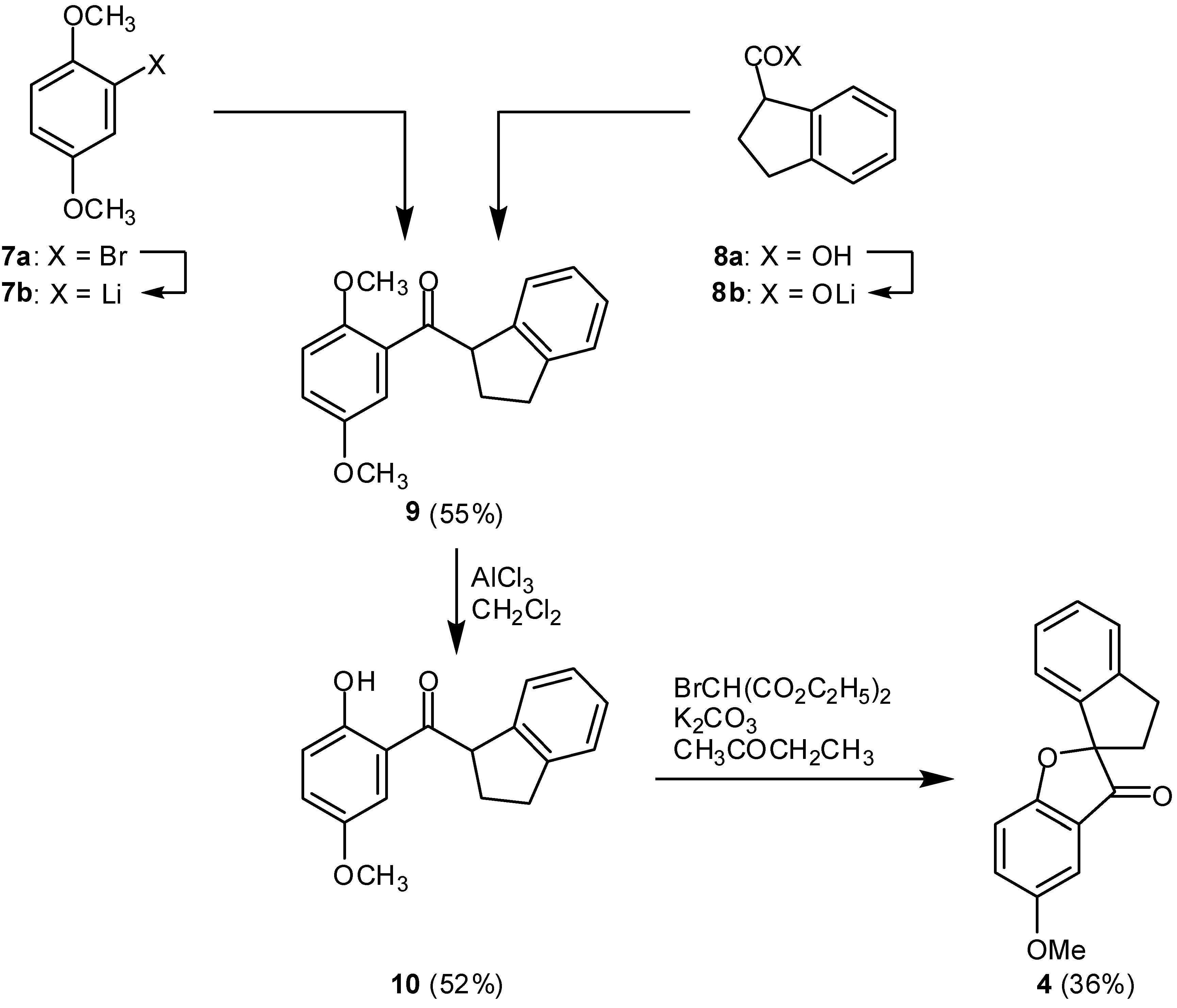
(2,3-Dihydro-1H-inden-1-yl) (2,5-dimethoxyphenyl)methanone (9):
Under nitrogen, a solution of 7a (6.015 g, 27.27 mmol) in dry diethyl ether (60 mL) was stirred at ‑78°C in a two-necked flask, equipped with a magnetic stirrer, a septum and a connection to the nitrogen/vacuum line. A solution of n-butyllithium (19.8 mL, 31.6 mmol) was added slowly by syringe, and stirring was continued at the same temperature for 30 min. In a second flask, 1-indanecarboxylic acid (8a, 2.25 g, 14.0 mmol) was dissolved in dry diethyl ether (30 mL), and treated at –78°C under stirring with a solution of n-butyllithium (8.6 mL, 13.8 mmol). Stirring was continued at 20°C for 30 min. The solution of the first flask was added through a cannula, and the mixture was allowed to reach room temperature overnight. Water (20 mL) was added, followed by 2 N hydrochloric acid (3 mL) and the aqueous layer was extracted three times with diethyl ether. The combined organic layers were dried with magnesium sulfate, the solvent was removed in a rotary evaporator and the residue was purified by column chromatography to give colorless, oily 9 (1,68 g, 55%); Rf = 0.36 (chloroform); 1H-NMR: δ 2.32-2.52 (m, 2H, CH2), 2.88-2.99 (m, 2H, CH2), 3.76 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 5.12 (dd, J = 8.5 Hz, J = 5.8 Hz, 1H, COCH), 6.95 (d, J = 8.9 Hz, 1H, 3-H), 7.04 (dd, J = 8.9 Hz, J = 3.2 Hz, 1H, 4-H), 7.09 (d, J = 3.2 Hz, 1H, 6-H), 7.05-7.26 (m, 4H, indenyl aromatic H); 13C-NMR: δ 29.5, 32.5, 56.9, 57.2, 58.6, 113.6, 115.1, 119.7, 125.1, 125.6, 126.6, 127.6, 129.6, 142.6, 145.1, 153.0, 154.0, 203.9; IR (thin film, cm–1): 2940, 1675, 1495, 1465, 1410, 1280, 1225, 815, 755; MS (70 eV): m/z (%): 282 (4, [M]+), 165 (100), 150 (6), 122 (7), 115 (10), 92 (6), 77 (10).
2-(2,3-Dihydro-1H-inden-1-ylcarbonyl)-4-methoxyphenol (10):
In a 250-mL flask equipped with a drying tube, 9 (1.68 g, 7.64 mmol) was dissolved in dichloromethane and cooled to 0°C. Aluminum chloride (5 g, 37.5 mmol) were added, and stirring was continued at 0°C for 1 h and at room temperature for another 70 min. Water (20 mL) and 2 N hydrochloric acid (20 mL) were added carefully. The organic layer was separated and the aqueous phase was extracted three times with chloroform. The combined organic layers were dried with magnesium sulfate, the solvent was removed under reduced pressure, and the residue was purified by column chromatography to give crystalline, yellow 10 (1.06, 52%); Rf = 0.59 (chloroform); mp. 105-106°C; 1H-NMR: δ 2.41−2.56 (m, 2H CΗ2), 2.98-3.24 (m, 2H, CH2), 3.82 (s, 3H, OCH3), 5.03 (t, J = 7.7 Hz, 1H, COCH), 6.98 (d, J = 9.1 Hz, 1H, 6-H), 7.17 (dd, J = 9.1 Hz, J = 3.0 Hz, 1H, 5-H), 7.39 (d, J = 3.0 Hz, 1H, 3-H), 7.10-7.33 (m, 4H, indenyl aromatic H), 12.0 (s, 1H, OH); 13C-NMR: δ 30.0, 32.0, 52.2, 56.0, 113.4, 118.6, 119.6, 124.3, 124.9, 125.0, 126.5, 127.7, 141,0, 144.7, 151.8, 157.8, 206.3; IR (thin film, cm–1): 3060, 3006, 2939, 2846, 1644, 1615, 1481, 1418, 1353, 1242, 1212, 1165, 1039, 829, 788, 753; MS (70 eV): m/z (%): 268 (21, [M]+), 151 (100), 117 (12), 115 (13), 95 (5). Anal. calcd. for C17H16O3: C, 76.10; H, 6.01. Found C, 75.82; H, 5.98.
5-Methoxy-2’,3’-dihydro-3H-spiro[1-benzofuran-2,1’-indene]-3-one (4):
Under nitrogen, a mixture of 10 (57.6 mg, 0.215 mmol), diethyl bromomalonate (77 mg, 0.322 mmol), anhydrous potassium carbonate (89 mg, 0.645 mmol) and dry butanone were refluxed for 9 h. The mixture was filtered and the residue was washed with butanone. The combined filtrates were evacuated and the residue was submitted to a column chromatography. The yellowish crude product was recrystallized from ethanol to give 4 in 36% yield (20.5 mg); Rf = 0.41 (chloroform); mp 129°C; 1H-NMR: δ 2.45 (ddd, J = 13.8 Hz, J = 4.7 Hz, J = 8.5 Hz, 1H, CHH), 2.67 (ddd, 1H, J = 13.8 Hz, J = 6.5 Hz, J = 8.5 Hz, 1H, CHH), 3.15-3.33 (m, 2H, CH2), 3.83 (s, 3H, OCH3), 6.89 (d, J = 7.7 Hz, 1H), 6.96 (d, 1H, J = 9.0 Hz), 7.04 (d, J = 2.8 Hz, 1H), 7.09 (pseudo-t, J = 6.8 Hz, 1H), 7.20 (m, 1H), and 7.27 (m, 1H) (aromatic H); 13C-NMR: δ 30.6, 35.9, 55.9, 98.9, 104.3, 114.3, 120.2, 123.2, 125.2, 127.2, 128.3, 129.8, 140.1, 145.6, 155.0, 167.4, 202.8; IR (thin film, cm–1): 3070, 2921, 2851, 1708, 1604, 1551, 1481, 1430, 1341, 1263, 1206, 1158, 1095, 1026, 998, 938, 915, 836, 801, 755, 652; MS (70 eV): m/z (%): 266 (56, [M]+), 151 (100), 123 (11), 115 (24), 32 (68); HRMS: (EI) cald. for C17H14O3: 266.0943, found 266.0940.