Silica Sulfuric Acid Promotes Aza-Michael Addition Reactions under Solvent-Free Condition as a Heterogeneous and Reusable Catalyst
Abstract
:Introduction
Results and Discussion
Experimental
General
General procedure for the synthesis of β-amino carbonyl compounds
Selected spectroscopic data
Conclusions
Acknowledgements
References
- Azizi, N.; Saidi, M.R. Novel and efficient method for the silylation of hydroxyl groups with hexamethyldisilazane (HMDS) under solvent-free and neutral conditions. Organometallics 2004, 23, 1457–1458. [Google Scholar] [CrossRef]
- Azizi, N.; Saidi, M.R. LiClO4 accelerated Michael addition of amines to α,β- unsaturated olefins under solvent-free conditions. Tetrahedron 2004, 60, 383–387. [Google Scholar] [CrossRef]
- Azizi, N.; Saidi, M.R. Lithium perchlorate–catalyzed three–component coupling: A facile and general method for the synthesis of α-aminophosphonates under solvent-free condition. Eur. J. Org. Chem. 2003, 4630–4633. [Google Scholar] [CrossRef]
- Azizi, N.; Saidi, M.R. Highly chemoselective addition of amines to epoxides in water. Org. Lett. 2005, 7, 3649–3651. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, L.W.; Zhou, W.; Li, L.; Xia, C.G. Highly efficient aza-Michael reactions of aromatic amines and N-heterocycles catalyzed by a basic ionic liquid under solvent-free conditions. Tetrahedron Lett. 2006, 47, 7723–7726. [Google Scholar] [CrossRef]
- Krishna, P. R.; Sreeshailam, A.; Srinivas, R. Recent advances and applications in asymmetric aza-Michael addition chemistry. Tetrahedron 2009, 65, 9657–9672. [Google Scholar] [CrossRef]
- Bartoli, G.; Bosco, M.; Marcantoni, E.; Petrini, M.; Sambri, L.; Torregiani, E. Conjugate addition of amines to α,β-enones promoted by CeCl3·7H2O−NaI system supported in silica gel. J. Org. Chem. 2001, 66, 9052–9055. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Banik, B.K. Bismuth nitrate-catalyzed versatile Michael reactions. J. Org. Chem. 2003, 68, 2109–2114. [Google Scholar] [CrossRef] [PubMed]
- Varala, R.; Alam, M.M.; Adapa, S.R. Chemoselective michael type addition of aliphatic amines to α,β-ethylenic compounds using bismuth triflate catalyst. Synlett 2003, 720–722. [Google Scholar] [CrossRef]
- Saidi, M.R.; Pourshojaei, Y.; Fezzeh, A. Highly efficient michael addition reaction of amines catalyzed by silica-supported aluminum chloride. Synthetic Commun. 2009, 39, 1109–1119. [Google Scholar] [CrossRef]
- Yadav, J.S.; Ramesh Reddy, A.; Gopal Rao, Y.; Narsaiah, A.V.; Reddy, B.V.S. Lanthanum trichloride (LaCl3): An efficient catalyst for conjugate addition of amines to electron-deficient olefins. Lett. Org. Chem. 2007, 4, 462–464. [Google Scholar]
- Khan, A.T.; Parvin, T.; Gazi, S.; Choudhury, L.H. Bromodimethyl sulfoniumbromide-mediated Michael addition of amines to electron deficient alkenes. Tetrahedron Lett. 2007, 48, 3805–3808. [Google Scholar] [CrossRef]
- Mukherjee, C.; Misra, A.K. Aza-Michael addition of amines to activated alkenes catalyzed by silica supported perchloric acid under a solvent-free condition. Lett. Org. Chem. 2007, 4, 54–59. [Google Scholar] [CrossRef]
- Duan, Z.; Xuan, X.; Li, T.; Yang, C.; Wu, Y. Cerium(IV) ammonium nitrate (CAN) catalyzed aza-Michael addition of amines to α,β-unsaturated electrophiles. Tetrahedron Lett. 2006, 47, 5433–5436. [Google Scholar] [CrossRef]
- Varala, R.; Sreelatha, N.; Adapa, S.R. Ceric ammonium nitrate catalyzed aza-Michael addition of aliphatic amines to α,β-unsaturated carbonyl compounds and nitriles in water. Synlett 2006, 1549–1553. [Google Scholar] [CrossRef]
- Surendra, K.; Krishnaveni, N.S.; Sridhar, R.; Rao, K.R. b-Cyclodextrin promoted aza-Michael addition of amines to conjugated alkenes in water. Tetrahedron Lett. 2006, 47, 2125–2127. [Google Scholar] [CrossRef]
- Meshram, H.M.; Lakshindra, C.; Reddy, P.N.; Sadashiv, K.; Yadav, J.S. Zirconium(IV) chloride–mediated chemoselective conjugate addition of aliphatic amines to α,β-ethylenic compounds. Synth. Commun. 2006, 36, 795–801. [Google Scholar] [CrossRef]
- Yadav, J.S.; Ramesh Reddy, A.; Gopal Rao, Y.; Narsaiah, A.V.; Subba Reddy, B.V. Samarium(III) triflate catalyzed conjugate addition of amines to electron-deficient alkenes. Synthesis 2007, 3447–3450. [Google Scholar] [CrossRef]
- Hashemi, M.M.; Eftekhari-Sis, B.; Abdollahifar, A.; Khalili, B. ZrOCl2 ·8H2O on montmorillonite K10 accelerated conjugate addition of amines to a,b-unsaturated alkenes under solvent-free conditions. Tetrahedron 2006, 62, 672–677. [Google Scholar] [CrossRef]
- Bo, K.L.; Wu, Q.; Xue, Q.Q.; De, S.L.; Xian, F.L. N-methyl-imidazole as a promising catalyst for the aza-Michael addition reaction of N-heterocycles. Synthesis 2007, 2653–2659. [Google Scholar]
- Kantam, M.L.; Neeraja, V.; Kavita, B.; Neelima, B.; Chaudhuri, M.K.; Hussain, S. Cu(acac)2 immobilized in ionic liquids: A recoverable and reusable catalytic system for aza-Michael reactions. Adv. Synth. Catal. 2005, 347, 763–766. [Google Scholar] [CrossRef]
- Yeom, C.E.; Kim, M.J.; Kim, B.M.; Choudhury, L.H. 1,8-Diazabicyclo [5.4.0]undec-7-ene (DBU)-promoted efficient and versatile aza-Michael addition. Tetrahedron 2007, 63, 904–909. [Google Scholar] [CrossRef]
- Monfray, J.; Koskinen, A.M.P. Aza-Michael additons on a,b-unsaturated esters catalyzed by bismuth(III) triflate in conventional chemistry and under microwave irradiation. Lett. Org. Chem. 2006, 3, 324–327. [Google Scholar] [CrossRef]
- Vijender, M.; Kishore, P.; Satyanarayana, B. Cadmium chloride (CdCl2): An efficient catalyst for conjugate addition of amines to electron-poor alkenes. Synth. Commun. 2007, 37, 591–594. [Google Scholar] [CrossRef]
- Bhanushali, M.J.; Nandurkar, N.S.; Jagtap, S.R.; Bhanage, B.M. Y(NO3)3·6H2O catalyzed aza-Michael addition of aromatic/hetero or aromatic amines under solvent-free conditions. Catal. Commun. 2008, 9, 1189–1195. [Google Scholar] [CrossRef]
- Reddy, K.R.; Kumar, N.S. Cellulose-supported copper(0) catalyst for aza-Michael addition. Synlett 2006, 2246–2250. [Google Scholar] [CrossRef]
- Pore, D.M.; Soudagar, M.S.; Desai, U.V. Potassium phosphate or silica sulfuric acid catalyzed conjugate addition of thiols to alpha,beta-unsaturated ketones at room temperature under solvent-free conditions. Tetrahedron Lett. 2006, 47, 9325–9328. [Google Scholar] [CrossRef]
- Minakata, S.; Komatsu, M. Organic Reactions on Silica in Water. Chem. Rev. 2009, 109, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Salehi, P.; Zolfigol, M.A.; Shirini, F.; Baghbanzadeh, M. silica sulfuric acid and silica chloride as efficient reagents for organic reactions. Curr. Org. Chem. 2006, 10, 2171–2189. [Google Scholar] [CrossRef]
- Shaabani, A.; Sarvary, A.; Rahmati, A.; Rezayan, A.H. ionic liquid/silica sulfuric acid promoted fast synthesis of a Biginelli-Like scaffold reaction. Lett. Org. Chem. 2007, 4, 68–71. [Google Scholar] [CrossRef]
- Li, J.T.; Dai, H.G.; Xu, W.Z.; Li, T.S. Michael addition of indole to α,β-unsaturated ketones catalysed by silica sulfuric acid under ultrasonic irradiation. J. Chem. Res. 2006, 41–42. [Google Scholar] [CrossRef]
- Wu, H; Shen, Y; Fan, L.Y.; Wan, Y; Zhang, P; Chen, C.F.; Wang, W.X. Stereoselective synthesis of β-amino ketones via direct Mannich-type reaction catalyzed with silica sulfuric acid. Tetrahedron 2007, 63, 2404–2408. [Google Scholar] [CrossRef]
- Shirini, F.; Zolfigol, M.A.; Mohammadi, K. Silica sulfuric scid as a mild and efficient reagent for the acetylation of alcohols in solution and under solvent free conditions. Bull. Korean Chem. Soc. 2004, 25, 325–327. [Google Scholar]
- Modarresi, A.R.; Nasrollahzadeh, M.; Khamooshi, F. Solvent-free preparation of primary carbamates using silica sulfuric acid as an efficient reagent. ARKIVOC 2007, xvi, 238–245. [Google Scholar]
Sample Availability: Contact the authors. |


| Entry | Amine | Michael acceptor | Product | Time (h) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | 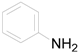 | PTEA | 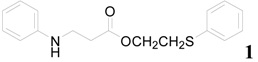 | 0.5 | 92 |
| 2 | 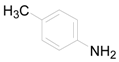 | PTEA | 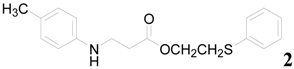 | 1.5 | 88 |
| 3 | 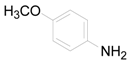 | PTEA | 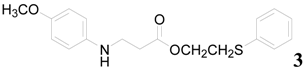 | 0.5 | 90 |
| 4 | 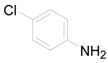 | PTEA | 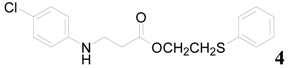 | 1.0 | 96 |
| 5 |  | PTEA | 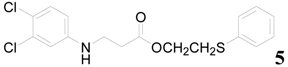 | 1.5 | 85 |
| 6 | 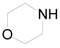 | PTEA | 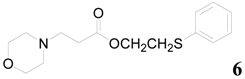 | 0.5 | 95 |
| 7 | 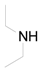 | PTEA | 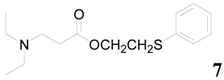 | 1.5 | 93 |
| 8 |  | PTEA | 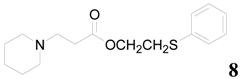 | 1.5 | 88 |
| 9 | 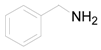 | PTEA | 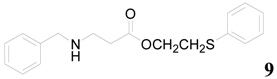 | 2.0 | 86 |
| 10 | 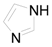 | PTEA |  | 4.0 | 12 |
| 11 | 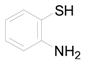 | PTEA | 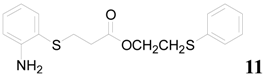 | 0.5 | 94 |
| 12 | 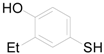 | PTEA | 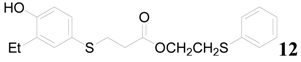 | 0.5 | 91 |
| 13 | 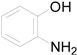 | PTEA | No Reaction | ||
| 14 | 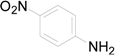 | PTEA | No Reaction | ||
| 15 | 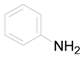 | PEEA |  | 2.5 | 86 |
| 16 |  | PEEA | 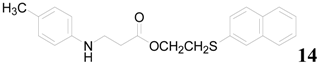 | 3.0 | 90 |
| 17 | 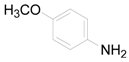 | PEEA | 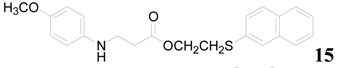 | 1.0 | 89 |
| 18 | 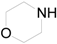 | PEEA |  | 0.5 | 92 |
| Entry | Solvent | Yield (%) |
|---|---|---|
| 1 2 3 4 5 6 7 8 | C2H5OH H2O CH3CO2H ClCH2CH2Cl Toluene CH3CN CH2Cl 2 Neat | 72 34 78 83 41 86 86 91 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Y.; Yuan, Y.-Q.; Guo, S.-R. Silica Sulfuric Acid Promotes Aza-Michael Addition Reactions under Solvent-Free Condition as a Heterogeneous and Reusable Catalyst. Molecules 2009, 14, 4779-4789. https://doi.org/10.3390/molecules14114779
Wang Y, Yuan Y-Q, Guo S-R. Silica Sulfuric Acid Promotes Aza-Michael Addition Reactions under Solvent-Free Condition as a Heterogeneous and Reusable Catalyst. Molecules. 2009; 14(11):4779-4789. https://doi.org/10.3390/molecules14114779
Chicago/Turabian StyleWang, Yan, Yan-Qin Yuan, and Sheng-Rong Guo. 2009. "Silica Sulfuric Acid Promotes Aza-Michael Addition Reactions under Solvent-Free Condition as a Heterogeneous and Reusable Catalyst" Molecules 14, no. 11: 4779-4789. https://doi.org/10.3390/molecules14114779




