Structural Changes of Malt Proteins During Boiling
Abstract
:Introduction
Results and Discussion
Protein and amino acid analysis
| Baudin wort | Guangmai wort | |||
|---|---|---|---|---|
| before boiling | after boiling | before boiling | after boiling | |
| Protein content (g/L) | 1.261 | 1.010 | 1.257 | 1.080 |
| Amino Acids contents (mg/100g) | Baudin | Guangmai | ||
|---|---|---|---|---|
| before boiling | after boiling | before boiling | after boiling | |
| Asp | 2,905.22 | 2,265.34 | 3,016.70 | 2,904.14 |
| Glu | 11,031.11 | 9,220.12 | 15,814.40 | 12,341.07 |
| Ser | 2,173.03 | 1,772.21 | 3,165.78 | 2,441.63 |
| Gly | 2,311.71 | 1,856.19 | 3,077.18 | 2,456.20 |
| His | 1,254.79 | 1,005.42 | 1,800.81 | 1,106.94 |
| Arg | 2,124.30 | 1,878.54 | 3,426.25 | 2,278.13 |
| Thr | 1,911.44 | 1,463.98 | 2,775.93 | 1,827.74 |
| Ala | 1,627.47 | 1,474.37 | 2,091.46 | 1,991.73 |
| Pro | 4,247.83 | 3,509.62 | 7,777.08 | 4,969.18 |
| Tyr | 1,627.96 | 1,340.96 | 2,999.85 | 1,606.79 |
| Val | 2,033.13 | 1,723.44 | 3,348.11 | 2,134.61 |
| Met | 816.20 | 536.48 | 994.47 | 518.34 |
| Cys | 228.72 | 150.04 | 373.23 | 126.16 |
| Ile | 1,359.58 | 1,073.72 | 2,229.27 | 1,401.63 |
| Leu | 2,414.81 | 2,094.16 | 4,157.57 | 2,346.27 |
| Phe | 1,473.31 | 1,323.31 | 2,894.09 | 1,624.15 |
| Lys | 1,043.84 | 962.62 | 1,665.08 | 822.67 |
| Total content | 40,584.46 | 33,650.52 | 61,607.25 | 42,897.37 |
Emission fluorescence spectroscopy analysis and surface hydrophobicity (Ho) and SH contents



DSC thermal characteristics
| To (°C) | Td (°C) | ΔH (J/g) | ΔT1/2 (°C) | |
|---|---|---|---|---|
| Baudin wort boiled before | 112.3 | 116.62 | 0.65 | 5.45 |
| Baudin wort boiled after | 111.44 | 115.53 | 0.47 | 6.79 |
| Guangmai wort boiled before | 111.39 | 115.91 | 1.06 | 5.98 |
| Guangmai wort boiled after | 111.84 | 115.09 | 0.98 | 6.61 |
Electrophoretic separation


Gel filtration chromatography analysis of wort proteins


CD analysis
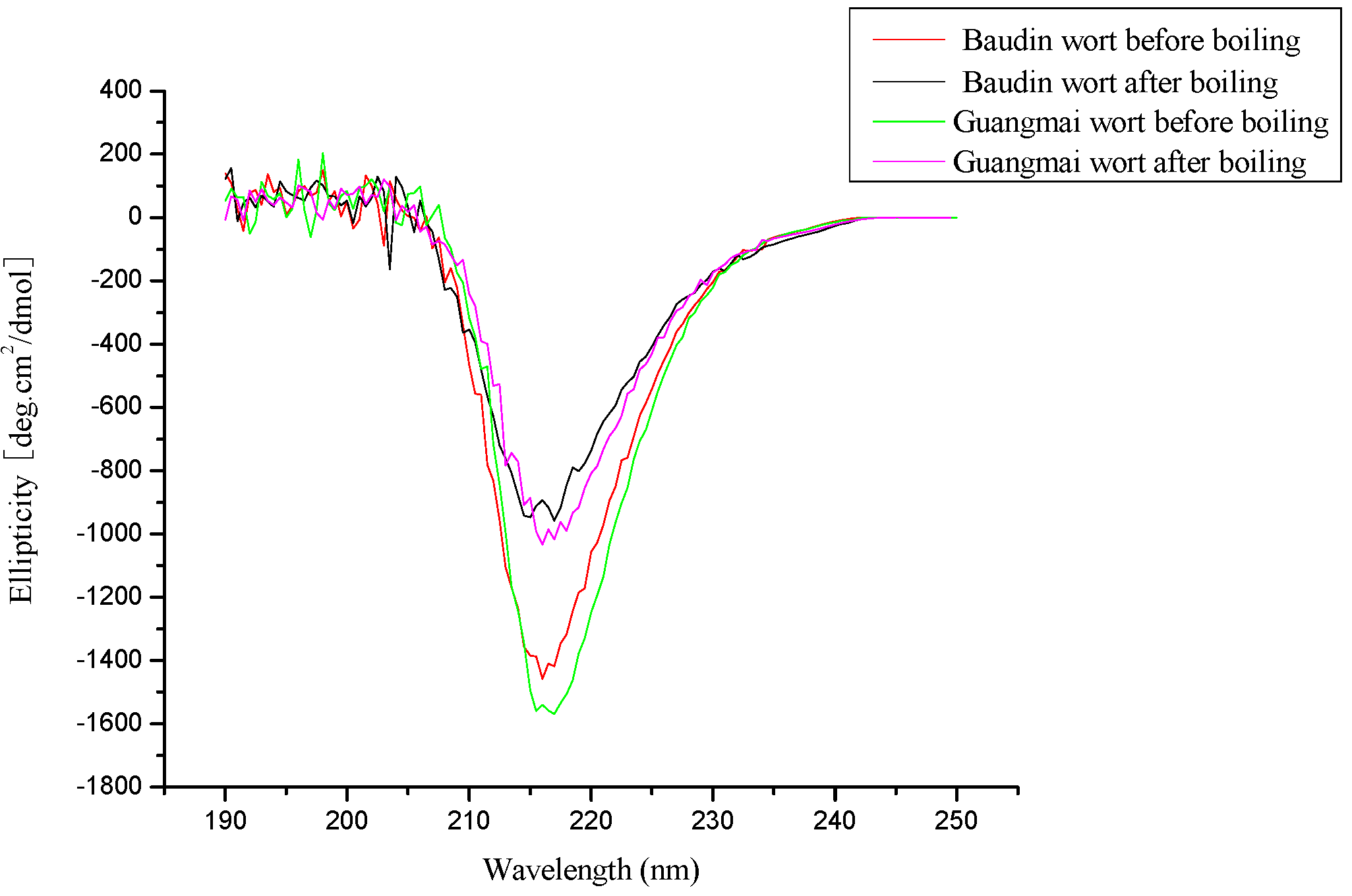
| Baudin wort | Guangmai wort | |||
|---|---|---|---|---|
| before boiling | after boiling | before boiling | after boiling | |
| α- helix (%) | 30.1 | 22.8 | 29.8 | 22.1 |
Conclusions
Experimental
Worts and preparation of wort protein
Analysis of amino acids
Measurement of surface hydrophobicity (Ho)
Measurement of free sulfhydryl (SH) content
Differential Scanning Calorimetry
Gel filtration chromatography
Electrophoretic analyses
Circular Dichroism Spectroscopy
Statistical analysis
Acknowledgments
References and Notes
- Ostergaard, O.; Melchior, S.; Roepstorff, P.; Svensson, B. Initial proteome analysis of mature barley seeds and malt. Proteomics 2002, 2, 733–739. [Google Scholar] [CrossRef]
- Janes, P.W.; Skerritt, J.H. High performance liquid chromatography of barley proteins: relative quantities of hordein fractions correlate with malt extract. J. Inst. Brew. 1993, 99, 78–84. [Google Scholar]
- Poyri, S.; Mikola, M.; Strohm-Sontag, T.; Norja-Kaukovirta, A.; Home, S. The formation and hydrolysis of barley malt gel-protein under different mashing conditions. J. Inst. Brew. 2006, 108(2), 261–267. [Google Scholar]
- Marchylo, B.A.; Krucrr, L.E.; Hatcher, D. High performance liquid chromatography and eletrophoretic analysis of hordein during malting for two barley varieties of contrasting malting quality. J. Cereal Chem. 1986, 63(3), 219–231. [Google Scholar]
- Silva, F.; Nogueira, L.C.; Goncalves, C.; Ferreira, A.A.; Ferreira, I. M.P.L.V.O.; Teixeira, N. Electrophoretic and HPLC methods for comparative study of the protein fractions of malts, worts, and beers produced from Scarlett and Prestige barley (Hordeum vulgare L.) varieties. Food Chem. 2008, 106, 820–829. [Google Scholar] [CrossRef]
- Perrocheau, L.; Bakan, B.; Boivin, P.; Marion, D. Stability of barley and malt lipid transfer protein 1(LTP1) toward heating and reducing agents: Relationships with the brewing process. J. Agric. Food Chem. 2006, 54, 3108–3113. [Google Scholar] [CrossRef]
- Leiper, K.A.; Stewart, G.G.; Mckeown, I.P. Beer polypeptides and silica gel Part I. Polypeptides involved in haze formation. J. Inst. Brew. 2003, 109(1), 57–72. [Google Scholar]
- Curioni, A.; Pressi, G.; Furegon, L.; Peruffo, A.D.B. Major proteins of beer and their precursors in barley: Electrophoretic and immunological studies. J. Agric. Food. Chem. 1995, 43, 2620–2626. [Google Scholar] [CrossRef]
- Perrocheau, L.; Rogniaux, H.; Boivin, P.; Marion, D. Probing heat-stable water-soluble proteins from barley to malt and beer. Proteomics 2005, 5, 2849–2858. [Google Scholar] [CrossRef]
- Hejgaard, J. Origin of a dominant beer protein: Immunochemical identity with a β-amylase-associated protein from barley. J. Inst. Brew. 1977, 83, 94–96. [Google Scholar] [CrossRef]
- Yokoi, S.; Maeda, K.; Xiao, R.; Kamada, K.; Kamimura, M. Characterization of beer proteins responsible for the foam of beer. In Proc. Eur. Brew. Conv. 22nd Congress, Zurich, Switzerland; 1989; pp. 593–600. [Google Scholar]
- Sorensen, S.B.; Bech, L.M.; Muldbjerg, M.; Beenfeldt, T.; Breddam, K. Barley lipid transfer protein1 is involved in beer foam formation. Tech. Q. Master Brew. Assoc. Am. 1993, 30, 136–145. [Google Scholar]
- Asano, K.; Shinagawa, K.; Hashimoto, N. Characterization of haze-forming proteins of beer and their roles in chill haze formation. J. Am. Soc. Brew. Chem. 1982, 40, 147–154. [Google Scholar]
- Jegou, S.; Douliez, Paul-Jean; Molle, D.; Boivin, P.; Marion, D. Evidence of the glycation and denaturation of LTP1 malting and brewing process. J. Agric. Food. Chem. 2001, 49, 4942–4949. [Google Scholar] [CrossRef]
- Lewis, M.J.; Young, T.W. Brewing; Aspen Publishers: Gaithersberg, MD, USA, 2001. [Google Scholar]
- Narziss, L. Modern wort boiling. Proc. Inst. Brew. Conv. Somerset West, S. Afr. 1993, 195–212. [Google Scholar]
- Bech, L.M.; Vaag, P.; Heinemann, B.; Breddam, K. Through-out the brewing process barley lipid transfer protein1 (LTP1) is transformed into a more foam-promoting form. In Proceedings of Congress-European Brewery Convention, Helsinki, Finland, 1995; IRL Press: Oxford, UK, 1995; pp. 561–568. [Google Scholar]
- Vaag, P.; Bech, L.M.; Carmeron-Mills, V.; Svendsen, I. Characterization of a beer foam protein originating from barley. In Proceedings of Congress-European Brewery Convention, Cannes, France, 1999; IRL Press: Oxford, UK, 1999; pp. 157–166. [Google Scholar]
- Gorinstein, S.; Zemser, M.; Albores, F.V.; Ochoa, J.L.; Lopez, O.P.; Scheler, Ch.; Salnikow, J.; Belloso, O.M.; Trakhtenberg, S. Proteins and amino acids in beers, their contents and relationships with other analytical data. Food Chem. 1999, 67, 71–78. [Google Scholar] [CrossRef]
- Osman, A.M.; Coverdale, S.M.; Watson-Onley, K.; Bell, D.; Healy, P. The gel filtration chromatographic-profiles of proteins and peptides of wort and beer: Effects of processing- Malting, Mashing, Kettle Boiling, Fermentation and Filtering. J. Inst. Brew. 2003, 109(1), 45–51. [Google Scholar]
- Bak-Jensen, K.S.; Laugesen, S.; Roepstorff, P.; Svensson, B. Two-dimensional gel electrophoresis pattern (pH6-11) and identification of water-soluble barley seed and malt proteins by mass spectrometry. Proteomics 2004, 4, 728–742. [Google Scholar] [CrossRef]
- Mortz, E.; Krogh, T.N.; Vorum, H.; Gorg, A. Improved silver staining protocols for high sensitivity protein identification using matrix-assisted laser desorption/ionization-time of flight analysis. Proteomics 2001, 1, 1359–1363. [Google Scholar] [CrossRef]
- Apperson, K.; Birch, D.; Leiper, K.; Mckeown, I. Fluorescence studies of beer protein uptake by silica. Proc. SPIE 2001, 4252, 71–78. [Google Scholar]
- Stathopoulos, C.E.; Tsiami, A.A.; Schofield, J.D.; Dobraszczyk, B.J. Effect of heat on rheology, surface hydrophobicity and molecular mass distribution of glutens extracted from flours with different bread-making quality. J. Cereal Sci. 2008, 47, 134–143. [Google Scholar] [CrossRef]
- Harwalkar, V.R.; Ma, C.-Y. Thermal analysis: principles and applications. In Food proteins properties and characterization; Nakai, S., Modler, H.W., Eds.; Wiley VCH: New York, USA, 1996; pp. 405–427. [Google Scholar]
- Arntfield, S.D.; Murray, E.D. The influence of processing parameters on food protein functionality I. Differential scanning calorimetry as an indicator of protein denaturation. J. Can. Inst. Food Sci. Tech. 1981, 14, 289–294. [Google Scholar]
- Harwalkar, V.R.; Ma, C.-Y. Study of thermal properties of oat globulin by differential scanning calorimetry. J. Food Sci. 1987, 52, 394–398. [Google Scholar] [CrossRef]
- Privalov, P.L. Stability of proteins: Proteins which do not present a single cooperative system. Adv. Protein Chem. 1982, 35, 1–104. [Google Scholar] [CrossRef]
- Heigaard, J. Purification and properties of protein Z – a major antigenic beer protein of barley origin. Plant Physiol. 1982, 54, 174–182. [Google Scholar] [CrossRef]
- Kordialik-Bogacka, E.; Ambroziak, W. Investigation of foam-active polypeptides during beer fermentation. J. Sci. Food Agric. 2004, 84, 1960–1968. [Google Scholar] [CrossRef]
- Roberts, R.T. Glycoproteins and beer foam. Proc. Eur. Brew. Conv. 1975, 15, 453–464. [Google Scholar]
- Kato, A.; Nakai, S. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochim. Biophys. Acta 1980, 624, 13–20. [Google Scholar] [CrossRef]
- Chan, K.Y.; Wasserman, B.P. Direct colorimetric assay of free thiol groups and disulfide bonds in suspensions of solubilized and particulate cereal proteins. J. Cereal Chem. 1993, 29, 35–37. [Google Scholar]
- Meng, G.T.; Ching, K.M.; Ma, C.Y. Thermal aggregation of globulin from an indigenous Chinese legume, Phaseolus angularis (red bean). Food Chem. 2002, 79, 93–103. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Fox, G.P.; Onley-Watson, K.; Osman, A. Multiple linear regression calibrations for barley and malt protein based on the spectra of hordein. J. Inst. Brew. 2002, 108, 155–159. [Google Scholar] [CrossRef]
- Moll, M. Composition of barley and malt. In Brewing Science; Pollack, J.R.A., Ed.; Academic Press: London, UK, 1979; pp. 2–117. [Google Scholar]
- O’Rourke, T. The function of wort boiling. Brew. Inter. 2002, 2, 17–19. [Google Scholar]
- van Gameron, Y.M. Protein flow during wort production. Tech. Q. Master Brew. Assoc. Am. 1995, 32, 238–240. [Google Scholar]
- Sample Availability: Samples of wort proteins are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jin, B.; Li, L.; Liu, G.-Q.; Li, B.; Zhu, Y.-K.; Liao, L.-N. Structural Changes of Malt Proteins During Boiling. Molecules 2009, 14, 1081-1097. https://doi.org/10.3390/molecules14031081
Jin B, Li L, Liu G-Q, Li B, Zhu Y-K, Liao L-N. Structural Changes of Malt Proteins During Boiling. Molecules. 2009; 14(3):1081-1097. https://doi.org/10.3390/molecules14031081
Chicago/Turabian StyleJin, Bei, Lin Li, Guo-Qin Liu, Bing Li, Yu-Kui Zhu, and Liao-Ning Liao. 2009. "Structural Changes of Malt Proteins During Boiling" Molecules 14, no. 3: 1081-1097. https://doi.org/10.3390/molecules14031081




