Polyphenols with Antiulcerogenic Action from Aqueous Decoction of Mango Leaves (Mangifera indica L.)
Abstract
:Introduction
Results and Discussion
| Weight (g) | Control | AD |
|---|---|---|
| Corporal | 43.00 ± 0.63 | 43.00 ± 1.18 |
| Kidney | 0.59 ± 0.31 | 0.52 ± 0.02 |
| Liver | 2.00 ± 0.72 | 1.82 ± 0.09 |
| Heart | 0,23 ± 0.01 | 0.18 ± 0.02 |
| Lungs | 0.26 ± 0.02 | 0.23 ± 0.01 |
| Mortality | 0/10 | 0/10 |
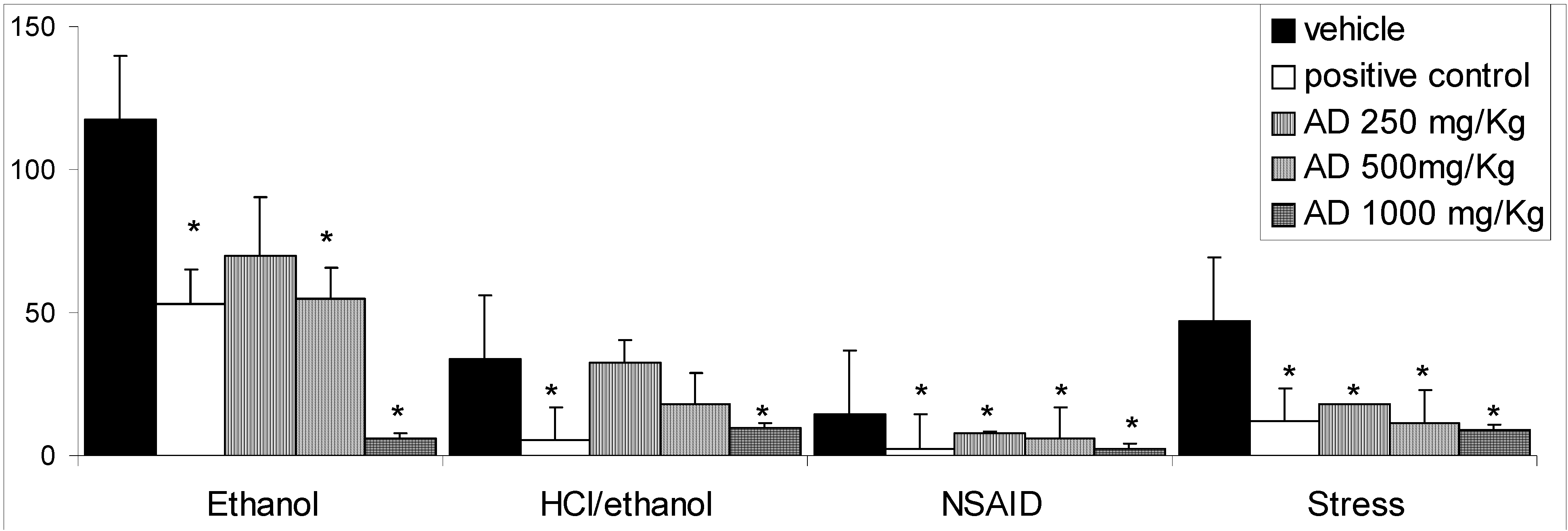
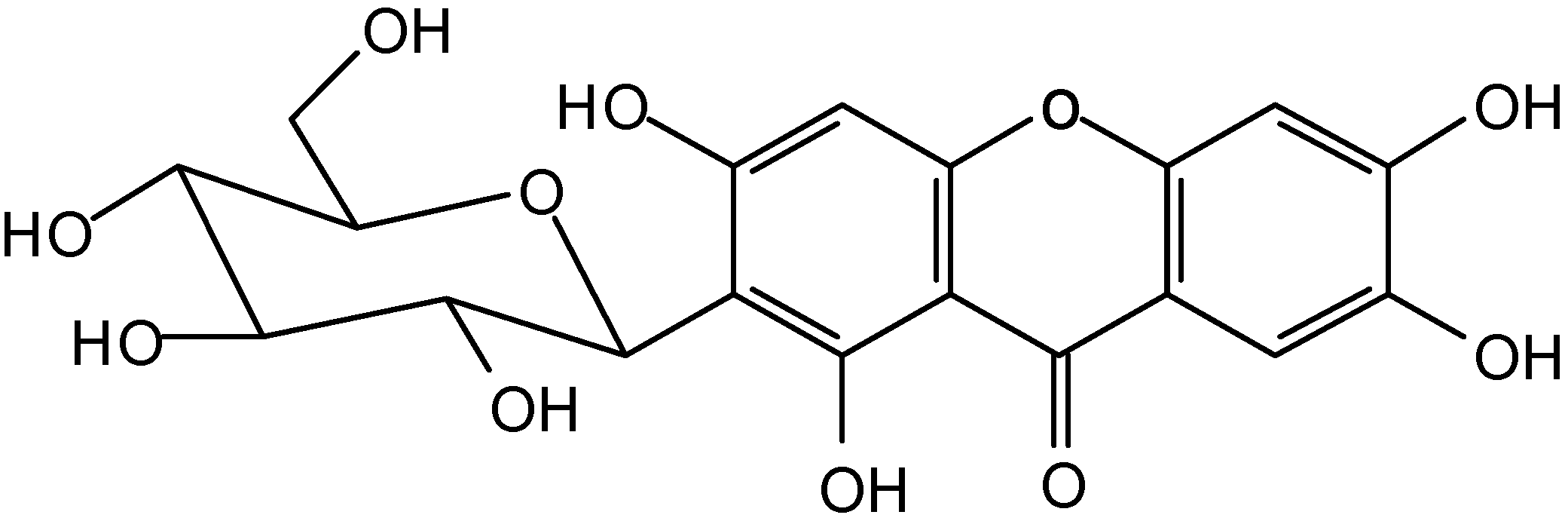

Conclusions
Experimental
General
Plant material, extraction and isolation
Quantitative determination of phenolic content in AD
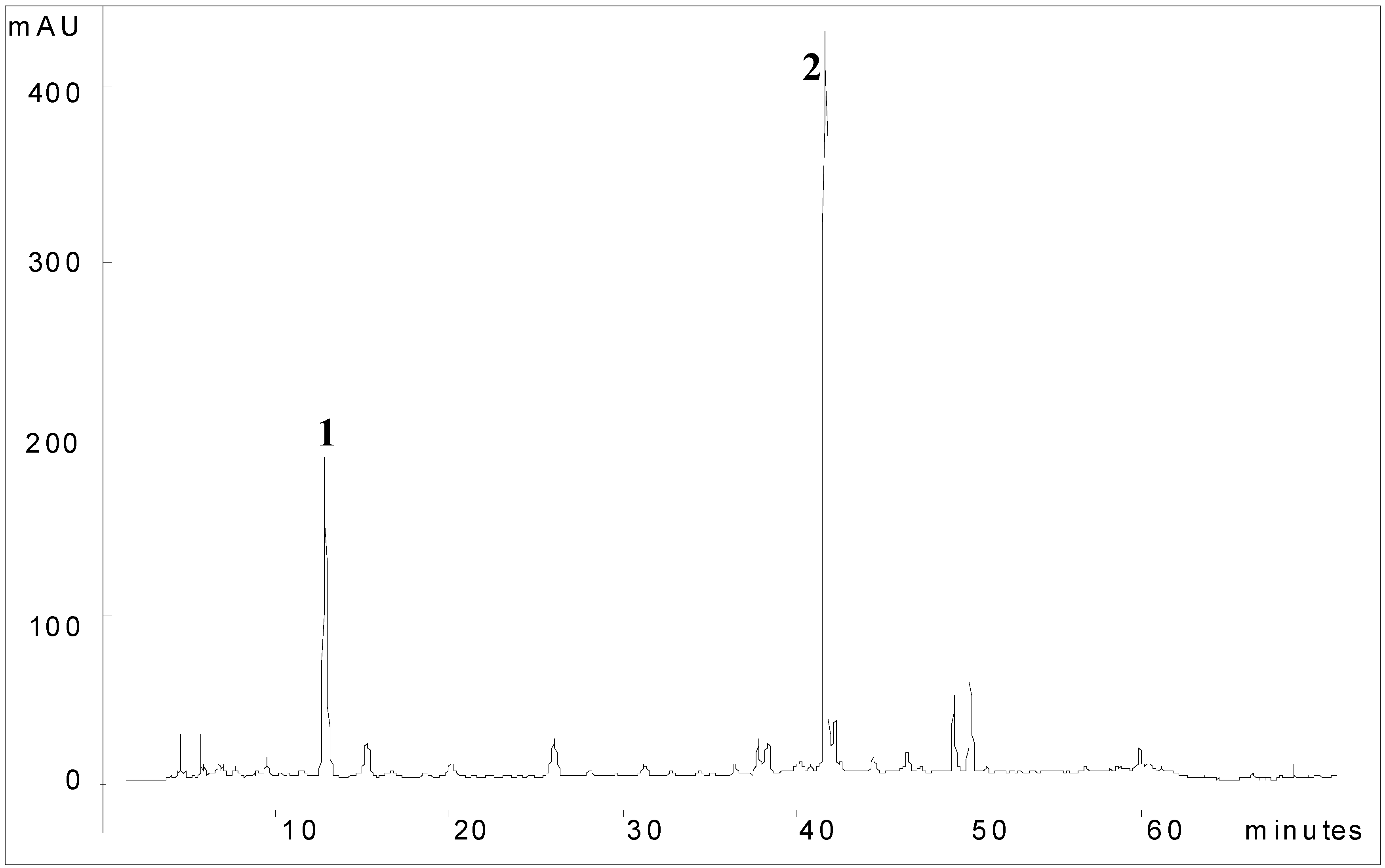
Chemical fingerprint of AD
Spectral data of compounds
Animals
Acute toxicity
Statistical analysis
Acknowledgements
References and Notes
- Qvistad, G.; Waldum, H. Rebound hypersecretion after inhibition of gastric acid secretion. Basic Clin. Pharmacol. Toxicol. 2004, 94, 202–208. [Google Scholar]
- Hirschowitz, B.I.; Keeling, D.; Lewin, M.; Okabe, S.; Parsons, M.; Sewing, K.; Wallmark, B.; Sachs, G. Pharmacological aspects of acid secretion. Dig. Dis. Sci. 1995, 40, 3–23. [Google Scholar] [CrossRef]
- Ross, I.A. Medicinal plants of the world; Human Press Inc.: New Jersey, USA, 1999; pp. 199–202. [Google Scholar]
- Robineau, L.G.; Soejarto, D.D. Medicinal Resources of the Tropical Forest; Balick, M.J., Elisabetsky, E., Laird, S.A., Eds.; Columbia University Press: New York, USA, 1996; pp. 318–325. [Google Scholar]
- Robineou, L.G. Hacia una Farmacopea Caribeña; Enda-Caribe: Santo Domingo, República Dominicana, 1995; pp. 351–353. [Google Scholar]
- Singh, U.P.; Singh, D.P.; Singh, M.; Maurya, S.; Srivastava, J.S.; Singh, R.B.; Singh, S.P. Characterization of phenolic compounds in some Indian mango cultivars. Int. J. Food Sci. Nutr. 2004, 55, 163–169. [Google Scholar] [CrossRef]
- Selles, N.A.J.; Castro, H.T.V.; Aguero-Aguero, J.; Gonzalez, J.; Nadeo, F.; De Simone, F.; Rastelli, L. Isolation and quantitative analysis of phenolic antioxidants, free sugars, and polyols from mango (Mangifera indica L) stem bark aqueous decoction used in Cuba as a nutritional supplement. J. Agric. Food Chem. 2002, 50, 762–766. [Google Scholar] [CrossRef]
- Anjaneyulu, V.; Babu, I.S.; Connollu, J.D. 29-hydroxymangiferonic acid from Mangifera indica. Phytochemistry 1994, 35, 1301–1303. [Google Scholar] [CrossRef]
- Kharn, M.A.; Nizami, S.S.; Khan, M.N.I.; Azeem, S.W.; Ahamed, Z. New triterpenes from Mangifera indica. J. Nat. Prod. 1994, 57, 988–991. [Google Scholar] [CrossRef]
- Saleh, N.A; El-Ansari, M.A. Polyphenolics of twenty local varieties of Mangifera indica. Planta Med. 1975, 28, 124–130. [Google Scholar] [CrossRef]
- Garrido, G.; Gonzalez, D.; Delporte, C.; Backhouse, N.; Quintero, G.; Nunez-Selles, A.J.; Morales, M.A. Analgesic and anti-inflammatory effects of Mangifera indica L. extract (Vimang). Phytother. Res. 2001, 15, 18–21. [Google Scholar] [CrossRef]
- Makare, N.; Bodhankar, S.; Rangari, V. Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice. J. Ethnopharmacol. 2001, 78, 133–137. [Google Scholar] [CrossRef]
- Garcia, D.; Delgado, R.; Ubeira, F.M.; Leiro, J. Modulation of rat macrophage function by the Mangifera indica L. extracts Vimang and mangiferin. Int. Immunopharmacol. 2002, 2, 797–806. [Google Scholar] [CrossRef]
- Garcia, D.; Leiro, J.; Delgado, R.; Sanmartin, M.L.; Ubeira, F.M. Mangifera indica L. extract (Vimang) and mangiferin modulate mouse humoral immune responses. Phytother. Res. 2003a, 17, 1182–1187. [Google Scholar] [CrossRef]
- Martinez, G.; Delgado, R.; Perez, G.; Garrido, G.; Nunez Selles, A.J.; Leon, O.S. Evaluation of the in vitro antioxidant activity of Mangifera indica L. extract (Vimang). Phytother. Res. 2000, 14, 424–427. [Google Scholar] [CrossRef]
- Sanchez, G.M.; Rodríguez, H.M.A.; Giuliani, A.; Núñez Sellés, A.J.; Rodríguez, N.P.; León Fernández, O.S.; Re, L. Protective effect of Mangifera indica L. extract (Vimang) on the injury associated with hepatic ischaemia reperfusion. Phytother. Res. 2003, 17, 197–201. [Google Scholar] [CrossRef]
- Sanchez, G.M.; Re, L.; Giuliani, A.; Nunez-Selles, A.J.; Davison, G.P.; Leon-Fernandez, O.S. Protective effects of Mangifera indica L. extract, mangiferin and selected antioxidants against TPA-induced biomolecules oxidation and peritoneal macrophage activation in mice. Pharmacol. Res. 2000, 42, 565–573. [Google Scholar] [CrossRef]
- Sairam, K.; Hemalatha, S.; Kumar, A.; Srinivasan, T.; Ganesh, J.; Shankar, M.; Venkataraman, S. Evaluation of anti-diarrhoeal activity in seed extracts of Mangifera indica. J. Ethnopharmacol. 2003, 84, 11–15. [Google Scholar] [CrossRef]
- Anila, L.; Vijayalakshmi, N.R. Flavonoids from Emblica officinalis and Mangifera indica-effectiveness for dyslipidemia. J. Ethnopharmacol. 2002, 79, 81–87. [Google Scholar] [CrossRef]
- Aderibigbe, A.O.; Emudianughe, T.S.; Lawal, B.A. Antihyperglycaemic effect of Mangifera indica in rat. Phytother. Res. 1999, 13, 504–507. [Google Scholar] [CrossRef]
- Aderibigbe, A.O.; Emudianughe, T.S.; Lawal, B.A. Evaluation of the antidiabetic action of Mangifera indica in mice. Phytother. Res. 2001, 15, 456–458. [Google Scholar] [CrossRef]
- Tona, L.; Kambu, K.; Ngimbi, N.; Mesia, K.; Penge, O.; Lusakibanza, M.; Cimanga, K.; De Bruyne, T.; Apers, S.; Totte, J.; Pieters, L.; Vlietinck, A.J. Antiamoebic and spasmolytic activities of extracts from some antidiarrhoeal traditional preparations used in Kinshasa, Congo. Phytomedicine 2000, 7, 31–38. [Google Scholar] [CrossRef]
- Garcia, D.; Escalante, M.; Delgado, R.; Ubeira, F.M.; Leiro, J. Anthelminthic and antiallergic activities of Mangifera indica L. stem bark components Vimang and mangiferin. Phytother. Res. 2003b, 17, 1203–1208. [Google Scholar]
- Bairy, I.; Reeja, S.; Siddharth, R.P.S.; Bhat, M.; Shivananda, P.G. Evaluation of antibacterial activity of Mangifera indica on anaerobic dental microflora based on in vivo studies. Indian J. Pathol. Microbiol. 2002, 45, 307–310. [Google Scholar]
- Lima, Z.P.; Severi, J.A.; Pellizzon, C.H.; Brito, A.R.M.S.; Solis, P.N.; Cáceres, A.; Girón, L.M.; Vilegas, W.; Hiruma-Lima, C.A. Can the aqueous decoction of mango flowers be used as an antiulcer agent? J. Ethnopharmacol. 2006, 106, 29–37. [Google Scholar] [CrossRef]
- Loomis, T.A.; Hayes, A.W. Essentials of Toxicology (4 edn); Academic Press Limited: London, 1996; pp. 33–46. [Google Scholar]
- Szabo, S.; Trier, J.S.; Frankel, P.W. Sulfhydryl compounds may mediate gastric cytoprotection. Science 1981, 214, 200–202. [Google Scholar]
- La Casa, C.; Villegas, I.; Alarcon de la Lastra, C.; Motilva, V.; Martin Balero, M.J. Evidence for protective and antioxidant properties of rutin, natural flavone, against ethanol induced gastric lesion. J. Ethnopharmacol. 2000, 71, 45–53. [Google Scholar] [CrossRef]
- Stadman, E.R.; Berlett, B.S. Free Radical Toxicology; Wallace, K.B., Ed.; Taylor & Francis: Bristol, USA, 1997; pp. 71–87. [Google Scholar]
- Morimoto, Y.; Shimohara, K.; Oshima, S.; Sukamoto, T. Effects of the new anti-ulcer agent KB-5492 on experimental gastric mucosal lesions and gastric mucosal defensive factors, as compared to those of teprenone and cimetidine. Jpn. J. Pharmacol. 1991, 57, 495–505. [Google Scholar] [CrossRef]
- Atay, S.; Tarnawski, A.S.; Dubois, A. Eicosanoids and the stomach. Prostaglandin their lipid mediat. 2000, 61, 105–124. [Google Scholar] [CrossRef]
- Takeuchi, K.; Furukawa, O.; Okada, M.; Niida, H.; Okabe, S. Influence of stress on gastric alkaline secretion in rats. J. Pharmacol. Exp.Ther. 1990, 252, 1228–1233. [Google Scholar]
- Blake, D.R.; Allen, R.E.; Lunee, J. Free radicals in biological systems- a review oriented to inflammatory process. Br. Med. Bull. 1987, 43, 371–385. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Role of free radicals and catalytic metal ions in human disease. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [CrossRef]
- Nishida, T.; Tsujii, M.; Tsujii, S. Are COX-2 inhibitors truly able to prevent NSAIDs- associated ulcers? Nippon Rinssho 2004, 62, 561–565. [Google Scholar]
- Gudis, K.; Sakamoto, C. The role of cycloxygenase in gastric mucosal protection. Dig. Dis.Sci. 2005, 50, s16–s23. [Google Scholar] [CrossRef]
- Shahat, A.A.; Hassan, R.A.; Nazif, N.M.; Miert, S.V.; Pieters, L.; Hammuda, F.M.; Vlietinck, A.J. Isolation of Mangiferin from Bombax malabaricum and structure revision of shaminin. Planta Med. 2003, 69, 1068–1070. [Google Scholar] [CrossRef]
- Repetto, M.G.; Lesuy, S.F. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz. J. Med. Biol. Res. 2002, 35, 523–534. [Google Scholar]
- Schieber, A.; Ullrich, W.; Carle, R. Characterization of polyphenols in mango puree concentrate by HPLC with diode array and mass spectrometric detection. Innov. Food Sci. Emerg. 2000, 1, 161–166. [Google Scholar] [CrossRef]
- Schieber, A.; Berardini, N.; Carle, R. Identification of flavonol and xanthone glycosides from mango (Mangifera indica L. Cv. “Tomy Atkins”) peels by High-Performance Liquid Chromatography-Electrospray Ionizations Mass Spectrometry. J. Agric. Food Chem. 2003, 51, 5006–5011. [Google Scholar] [CrossRef]
- Yoshimi, N.; Matsunaga, K.; Katayama, M.; Yamada, Y.; Kuno, T.; Qiao, Z.; Hara, A.; Yamahara, J.; Mori, H. The inhibitory effects of mangiferin, a naturally occurring glucosylxanthone, in bowel carcinogenesis of male F344 rats. Cancer Lett. 2001, 163, 163–170. [Google Scholar] [CrossRef]
- Zheng, M. S.; Lu, Z. Y. Antiviral effects of mangiferin and isomangiferin on Herpes simples virus. Chin. Med. J. 1990, 103, 160–165. [Google Scholar]
- Miura, T.; Ichiki, H.; Hashimoto, I.; Iwamoto, N.; Kato, M.; Kubo, M.; Ishihara, E.; Komatsu, Y.; Okada, M.; Ishida, T.; Tanigawa, K. Antidiabetic activity of a xanthone compound, mangiferin. Phytomedicine 2001, 8, 85–87. [Google Scholar] [CrossRef]
- Garrido, G.; González, D.; Lemus, Y.; García, D.; Lodeiro, L.; Quintero, G.; Delporte, C.; Núñez-Sellés, A.J.; Delgado, R. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (Vimang). Pharmacol. Res. 2004, 50, 143–149. [Google Scholar]
- Sato, T.; Kawamoto, A.; Tamura, A.; Tatsumi, Y.; Fujii, T. Mechanism of antioxidant action of pueraria glycoside (PG)-1 (an isoflavonoid) and mangiferin (a xanthonoid). Chem. Pharm. Bull. 1992, 721–724. [Google Scholar]
- Markus, B. Electrochemical behavior and antioxidant activity of some natural polyphenols. Helv. Chim. Acta 1996, 79, 1147–1158. [Google Scholar] [CrossRef]
- Carvalho, A.C.S.; Guedes, M.M.; Souza, A.L.; Trevisan, M.T.S.; Lima, A.F.; Santos, F.A.; Rao, V.S.N. Gastroprotective effect of mangiferin, a xanthonoid from Mangifera indica, against gastric injury induced by ethanol and indomethacin in rodents. Planta Med. 2005, 73, 1372–1376. [Google Scholar]
- Leiro, J. M.; Álvarez, E.; Arranz, J. A.; Siso, I. G.; Orallo, F. In vitro effects of mangiferin on superoxide concentrations and expression of the inducible nitric oxide synthase, tumor necrosis factor-α and transforming growth factor-β genes. Biochem. Pharmacol. 2003, 65, 1361–1371. [Google Scholar] [CrossRef]
- Andreu, G.P.; Delgado, R.; Velho, J.A.; Curti, C.; Vercesi, A. E. Iron complexing activity of mangiferin, a naturally occurring glucosylxanthone, inhibits mitochondrial lipid peroxidation induced by Fe2+-citrate. Eur. J. Pharmacol. 2005, 513, 47–55. [Google Scholar] [CrossRef]
- Baggett, S.; Mazzola, E.P.; Kennelly, E.J. The benzophenones: Isolation, structural elucidation and biological activities. Stud. Nat. Prod. Chem. 2005, 32, 721–771. [Google Scholar] [CrossRef]
- Venu, T.D.; Shashikanth, S.; Khanum, S.A.; Naveen, S.; Firdouse, A.; Sridhar, M.A.; Prasad, J.S. Synthesis and crystallographic analysis of benzophenone derivatives—The potential anti- inflammatory agents. Bioorg. Med. Chem. 2007, 15, 3500–3514. [Google Scholar]
- Prabhakar, B.T.; Khanum, S.A.; Jayashree, K.; Salimath, B.P.; Shashikanth, S. Anti-tumor and proapoptotic effect of novel synthetic benzophenone analogues in Ehrlich ascites tumor cells. Bioorg. Med. Chem. 2006, 14, 435–446. [Google Scholar] [CrossRef]
- Bakana, P.; Claeys, M.; Totte, J.; Pieters, L.A.; Van Hoof, L.; Tamba-Vemba, V.B.D.A.; Vlietinck, A.J. Structure and chemotherapeutical activity of a polyisoprenylated benzophenone from the stem bark of Garcinia huillensis. J. Ethnopharmacol. 1987, 21, 75–84. [Google Scholar] [CrossRef]
- Dos Santos, M. H. Estudo químico dos frutos de Rheedia gardneriana e efeito biológico de seus constituintes. Thesis, Universidade Federal de Viçosa, Minas Gerais, Brazil, 1996. [Google Scholar]
- Chatterjee, A.; Yasmin, T.; Bagchi, D.; Stohs, S.J. The bactericidal effects of Lactobacillus acidophilus, garcinol and protykin compared to clarithromycin, on Helicobacter pylori. Mol. Cell. Biochem. 2003, 243, 29–35. [Google Scholar] [CrossRef]
- Ma, Y.M.; Li, Y.; Liu, J.Y.; Song, Y.C.; Tan, R.X. Anti-Helicobacter pylori metabolites from Rhizoctonia sp. Cy064, an endophytic fungus in Cynodon dactylon. Fitoterapia 2004, 75, 451–456. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vit. 1965, 16, 144–158. [Google Scholar]
- Olfert, E.D.; Cross, B.M.; McWilliam, A.A. Guide to the Care and Use of Experimental Animals; Canadian Council on Animal Care Co.: Ontario, Canada, 1993; pp. 1–213. [Google Scholar]
- Souza Brito, A.R.M. Manual de Ensaios Toxicológicos in vivo; Editora da Unicamp: Campinas, São Paulo, Brazil, 1995; pp. 15–22. [Google Scholar]
- Mizui, T.; Doteuchi, M. Effect of polyamines on acidified ethanol-induced gastric lesions in rats. Jpn. J. Pharmacol. 1983, 33, 939–945. [Google Scholar] [CrossRef]
- Szelenyi, I.; Thiemer, K. Distention ulcer as a model for testing of drugs for ulcerogenic side effects. Arch. Toxicol. 1978, 41, 99–105. [Google Scholar] [CrossRef]
- Rainsford, K.D. Gastric ulcerogenicity of non-steroidal anti-inflammatory drug as in mice with mucosa sensitized by cholinomimetic treatment. Biochem. Pharmacol. 1978, 27, 1281–1289. [Google Scholar] [CrossRef]
- Levine, R.J. Peptic Ulcer; Pfeiffer, C.J., Ed.; Munksgaard: Copenhagen, Denmark, 1971; pp. 92–97. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the author.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Severi, J.A.; Lima, Z.P.; Kushima, H.; Monteiro Souza Brito, A.R.; Campaner dos Santos, L.; Vilegas, W.; Hiruma-Lima, C.A. Polyphenols with Antiulcerogenic Action from Aqueous Decoction of Mango Leaves (Mangifera indica L.). Molecules 2009, 14, 1098-1110. https://doi.org/10.3390/molecules14031098
Severi JA, Lima ZP, Kushima H, Monteiro Souza Brito AR, Campaner dos Santos L, Vilegas W, Hiruma-Lima CA. Polyphenols with Antiulcerogenic Action from Aqueous Decoction of Mango Leaves (Mangifera indica L.). Molecules. 2009; 14(3):1098-1110. https://doi.org/10.3390/molecules14031098
Chicago/Turabian StyleSeveri, Juliana Aparecida, Zeila Pinheiro Lima, Hélio Kushima, Alba Regina Monteiro Souza Brito, Lourdes Campaner dos Santos, Wagner Vilegas, and Clélia Akiko Hiruma-Lima. 2009. "Polyphenols with Antiulcerogenic Action from Aqueous Decoction of Mango Leaves (Mangifera indica L.)" Molecules 14, no. 3: 1098-1110. https://doi.org/10.3390/molecules14031098





