A New Insecticidal Sesquiterpene Ester from Celastrus Angulatus
Abstract
:1. Introduction
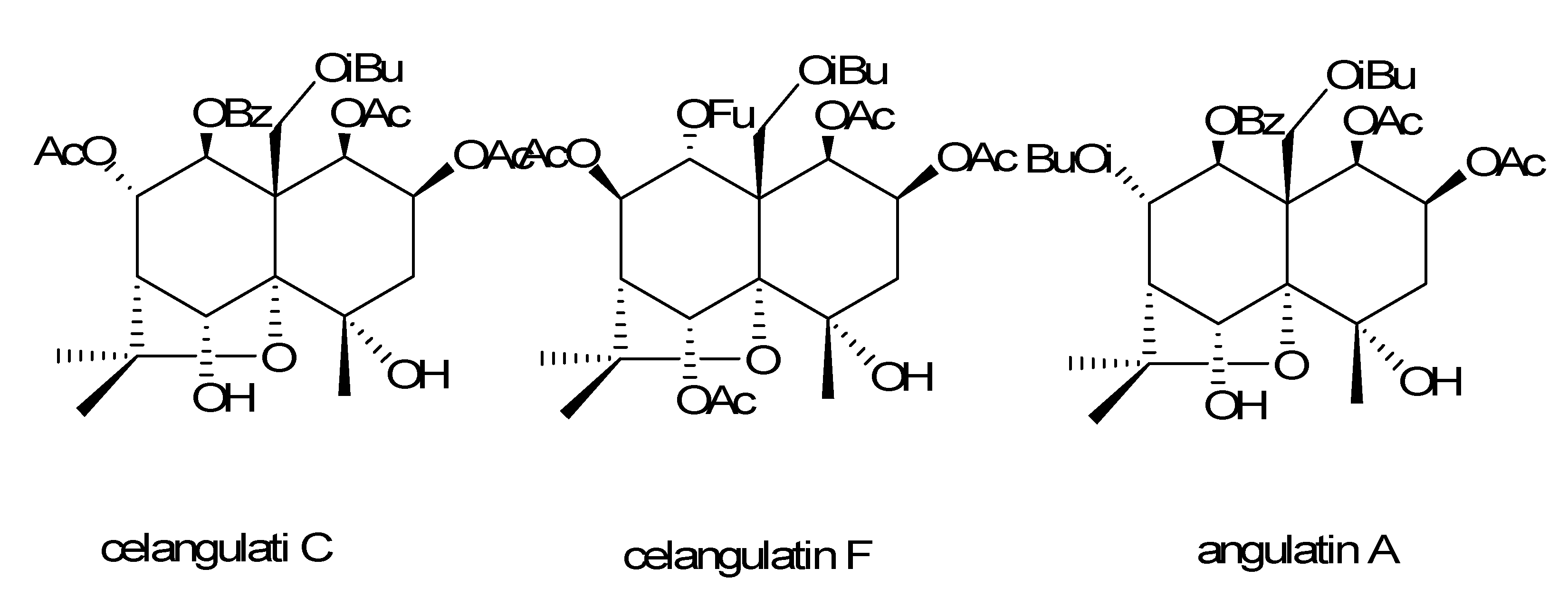
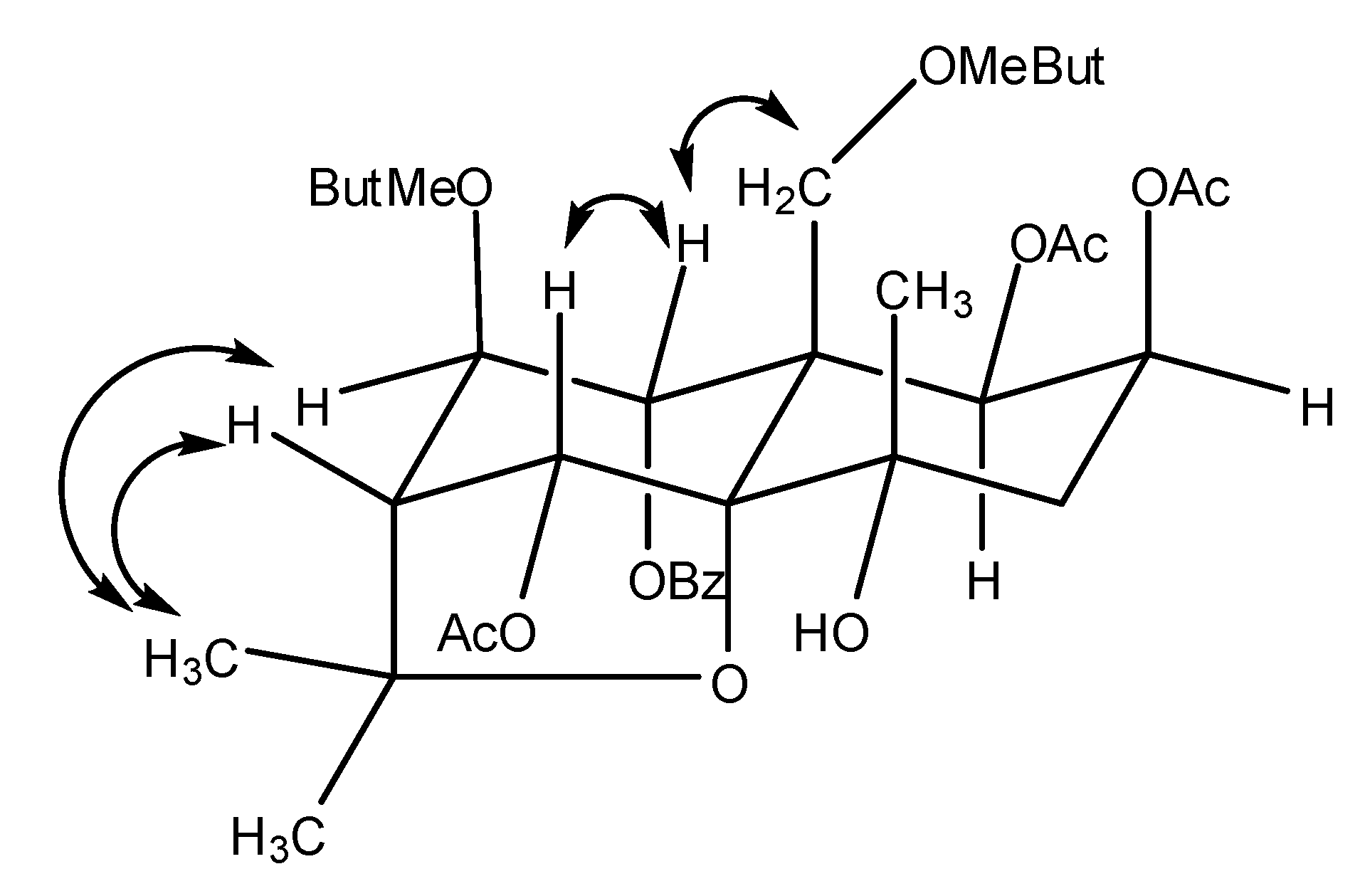
2. Results and Discussion
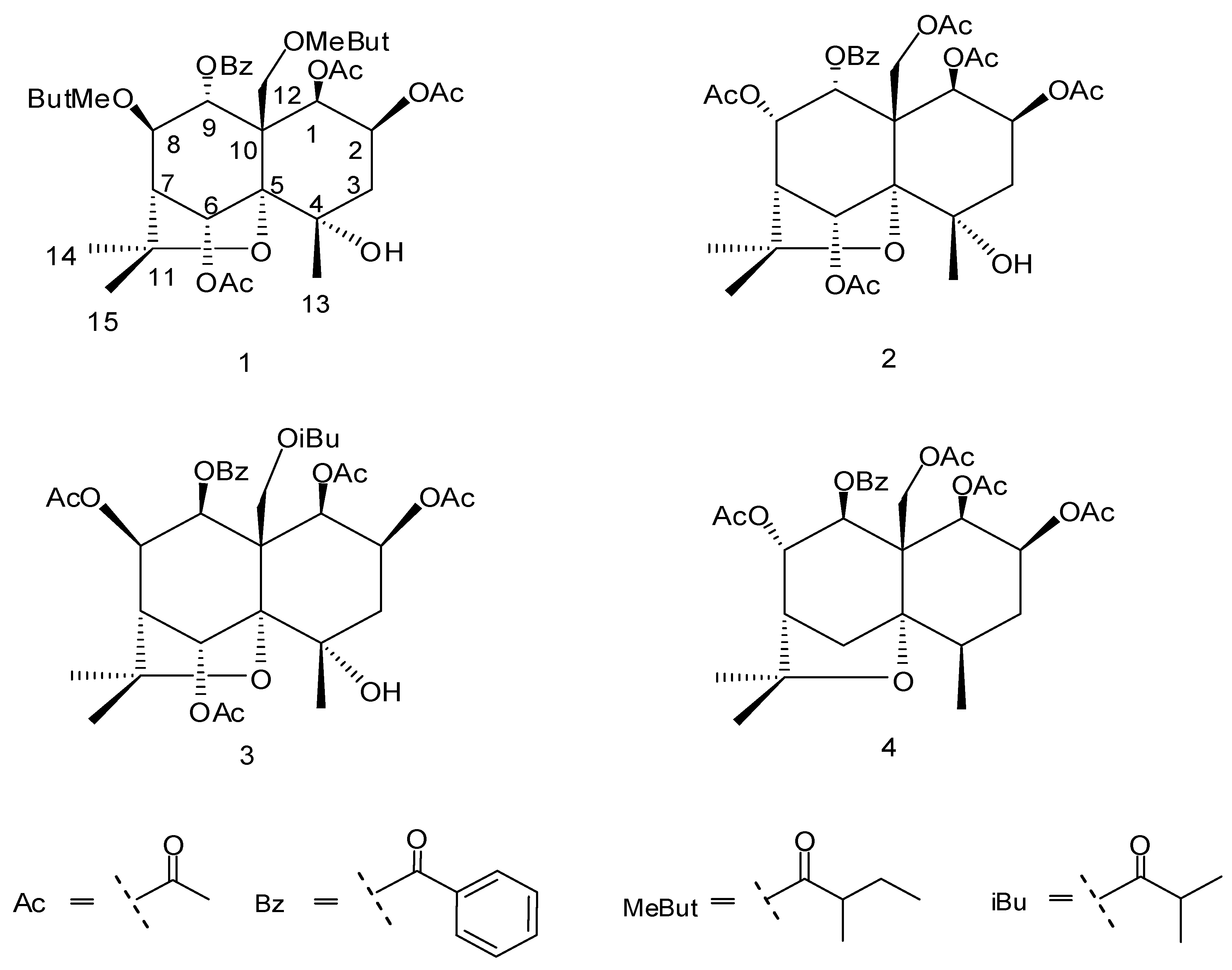
| No. | Δ C (DEPT) | δH (J, Hz) | HMBC |
|---|---|---|---|
| 1 | 71.09 CH | 5.62 (1H, d, J=3.5 Hz) | C-2,C-10, C=O of Ac |
| 2 | 68.26 CH | 5.56 (1H, dd, J=3.5 Hz, J=3.0 Hz) | C-10, C=O of Ac |
| 3 | 42.27 CH2 | 2.24 (1H, m), 2.00 (1H, m) | C-1,C-2,C-4,C-5,C-13 |
| 4 | 70.06 C | ||
| 5 | 91.64 C | ||
| 6 | 75.58 CH | 6.25 (1H,s) | C-5,C-7,C-8,C-10,C-11, C=O of Ac |
| 7 | 53.35 CH | 2.37 (1H, d, J=3.0 Hz) | C-5,C-6,C-8,C-9 |
| 8 | 76.32 CH | 5.32 (1H, d, J=3.0 Hz) | C=O of MeBut |
| 9 | 72.31 CH | 5.68 (1H, s) | C-5,C-7,C-8,C-10,C-12, C=O of Bz |
| 10 | 54.20 C | ||
| 11 | 83.69 C | ||
| 12 | 65.72 CH2 | 4.87 (1H,d, J=10.0 Hz) 4.83 (1H,d, J=10.0 Hz) | C-1,C-5,C-9,C-10, C=O of MeBut |
| 13 | 24.72 CH3 | 1.49 (3H, s) | C-4, C-5 |
| 14 | 25.83 CH3 | 1.65 (3H, s) | C-7, C-11 |
| 15 | 29.76 CH3 | 1.62 (3H, s) | C-7, C-11 |
| Ac | 169.85 (CO), 21.59 (CH3) | 2.10 (3H, s) | |
| Ac | 169.60 (CO), 21.28 (CH3) | 2.07 (3H, s) | |
| Ac | 169.47 (CO), 20.53 (CH3) | 1.46 (3H, s) | |
| MeBut | 176.68 (CO) 41.28 (CH), 26.65 (CH2), 11.82 (CH3), 16.68 (CH3) | 2.59 (1H, m), 1.80 (2H, m), 1.25 (3H, d, J=2.0 Hz), 0.96 (3H, m) | |
| MeBut | 175.42 (CO) 41.22 (CH), 26.58 (CH2), 11.67 (CH3), 16.49 (CH3) | 2.50 (1H, m), 1.55 (2H, m), 1.23 (3H, d, J=2.0 Hz), 0.96 (3H, m) | |
| Bz | 164.64 (CO), 133.96 (CH), 130.34 (2×CH), 128.60 (2×CH), 128.52 (C) | 8.00 (2H, d, J=7.0 Hz), 7.59 (1H, t, J=7.0 Hz), 7.45 (2H, t, J=7.0 Hz) |
| Compounds | KD50 (μg· g-1) |
|---|---|
| 1 | 252.3 |
| 2 | 290.1 |
| 3 | 360.2 |
| 4 | 884.3 |
| Celangulatin C | 280.4 |
| Celangulatin F | 201.5 |
| Angulatin A | 300.9 |
3. Experimental
3.1. General
3.2. Plant material
3.3. Extraction and isolation
3.4. Insecticidal activity
Acknowledgements
References
- Gao, J.M.; Wu, W.J.; Zhang, J.W.; Konishi, Y. The dihydro-β-agarofuran sesquiterpenoids. Nat. Prod. Rep. 2007, 24, 1153–1189. [Google Scholar] [CrossRef]
- Smith, R.G. The Celastraceae alkaloids. In The Alkaloids; Manske, R.H.F., Ed.; Academic Press: New York, 1977; Vol. XVI, pp. 215–248. [Google Scholar]
- Tu, Y.Q. Structures of two new sesquiterpenoid insect antifeedants from Celastrus rosthornianus. J. Chem. Soc. Perkin Trans. I 1991, 1, 425–427. [Google Scholar] [CrossRef]
- Takaishi, Y.; Ohshima, S.; Nakano, K.; Tomimatsu, T.; Tokuda, H.; Nishino, H.; Iwashima, A. Structures of sesquiterpene polyolesters from Celastrus stephanotiifolius with potential tumor-promotion inhibitory activity. J. Nat. Prod. 1993, 56, 815–824. [Google Scholar] [CrossRef]
- Kim, S.E.; Kim, Y.H.; Lee, J.J. A new sesquiterpene ester from Celastrus orbiculatus reversing multidrug resistance in cancer cells. J. Nat. Prod. 1998, 61, 108–111. [Google Scholar] [CrossRef]
- Kim, S.E.; Kim, H.S.; Hong, Y.S.; Kim, Y.C.; Lee, J.J. Sesquiterpene esters from Celastrus orbiculatus and their structure-activity relationship on the modulation of multidrug resistance. J. Nat. Prod. 1999, 62, 697–700. [Google Scholar] [CrossRef]
- Duan, H.; Takaishi, Y. Sesquiterpene evoninate alkaloids from Tripterygium hypoglaucum. Phytochemistry 1999, 52, 1735–1738. [Google Scholar] [CrossRef]
- Duan, H.; Takaishi, Y.; Bando, M.; Kido, M.; Imakura, Y.; Lee, K.H. Novel sesquiterpene esters with alkaloid and monoterpene and related compounds from Tripterygium hypoglaucum: a new class of potent anti-HIV agents. Tetrahedron Lett. 1999, 40, 2969–2972. [Google Scholar] [CrossRef]
- Duan, H.; Takaishi, Y.; Imakura, Y.; Jia, Y.; Li, D.; Cosentino, L.M.; Lee, K.H. Sesquiterpene alkaloids from Tripterygium hypoglaucum and Tripterygium wilfordii: a new class of potent anti-HIV agents. J. Nat. Prod. 2000, 63, 357–361. [Google Scholar] [CrossRef]
- Duan, H.; Takaishi, Y.; Imakura, Y.; Jia, Y.; Li, D. Immunosuppressive sesquiterpene alkaloids from Tripterygium wilfordii. J. Nat. Prod. 2001, 64, 582–587. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Huang, P.H. Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1999; Volume 45, pp. 7–128. [Google Scholar]
- Ji, Z.Q.; Hu, Z.N.; Liu, G.Q.; Wu, W.J. Advances in research of insecticidal components of Celastrus angulatus (Celastraceae). Acta Botanica Boreali-Occidentalia Sinica. 2004, 24, 748–753. [Google Scholar]
- Wu, W.J.; Wang, M.A.; Zhu, J.B.; Zhou, W.M.; Hu, Z.N.; Ji, Z.Q. Five new insecticidal sesquiterpenoids from Celastrus angulatus. J. Nat. Prod. 2001, 64, 364–367. [Google Scholar] [CrossRef]
- Wu, W.J.; Tu, Y.Q.; Zhu, J.B. Celangulin II, III and IV: newinsecticidal sesquiterpenoids from Celastrus angulatus. J.Nat. Prod. 1992, 55, 1294–1298. [Google Scholar] [CrossRef]
- Chen, C.Q.; Wu, D.G.; Liu, J.K. Angulatueoids A-D, Four Sesquiterpenes from the Seeds of Celastrus angulatus. Phytochemistry. 1992, 31, 2777–2780. [Google Scholar] [CrossRef]
- Wu, W.J.; Wang, M.A.; Zhou, W.M.,; Zhu, J.B.; Ji, Z.Q.; Hu, Z.N. Insecticidal sesquiterpene polyol esters from Celastrus angulatus. Phytochemistry. 2001, 58, 1183–1187. [Google Scholar] [CrossRef]
- Zhu, J.B.; Wang, M.A.; Wu, W.J.; Ji, Z.Q.; Hu, Z.N. Insecticidal sesquiterpene pyridine alkaloids from Euonymus species. Phytochemistry. 2002, 61, 699–704. [Google Scholar] [CrossRef]
- Ji, Z.Q.; Wu, W.J.; Yang, H.; Shi, B.J.; Wang, M.A. Four novel insecticidal sesquiterpene esters from Celastrus angulatus. Nat. Prod. Res. 2007, 21, 334–342. [Google Scholar] [CrossRef]
- Wang, M.A.; Wu, W.J.; Zhu, J.B.; Ji, Z.Q.; Zhou, W.M. Two new insecticidal sesquiterpene polyol esters from Celastrus angulatus. Nat. Prod. Res. 2006, 20, 653–658. [Google Scholar] [CrossRef]
- Wang, K.; Pan, Y. A novel sesquiterpene polyol ester from the Celastrus rosthornianus with anti-tumor activities. Nat. Prod. Commun. 2006, 1, 537–539. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wei, S.-p.; Ji, Z.-q.; Zhang, J.-w. A New Insecticidal Sesquiterpene Ester from Celastrus Angulatus. Molecules 2009, 14, 1396-1403. https://doi.org/10.3390/molecules14041396
Wei S-p, Ji Z-q, Zhang J-w. A New Insecticidal Sesquiterpene Ester from Celastrus Angulatus. Molecules. 2009; 14(4):1396-1403. https://doi.org/10.3390/molecules14041396
Chicago/Turabian StyleWei, Shao-peng, Zhi-qin Ji, and Ji-wen Zhang. 2009. "A New Insecticidal Sesquiterpene Ester from Celastrus Angulatus" Molecules 14, no. 4: 1396-1403. https://doi.org/10.3390/molecules14041396




