Biotransformation of Ginsenoside Rf to Rh1 by Recombinant β-Glucosidase
Abstract
:Introduction
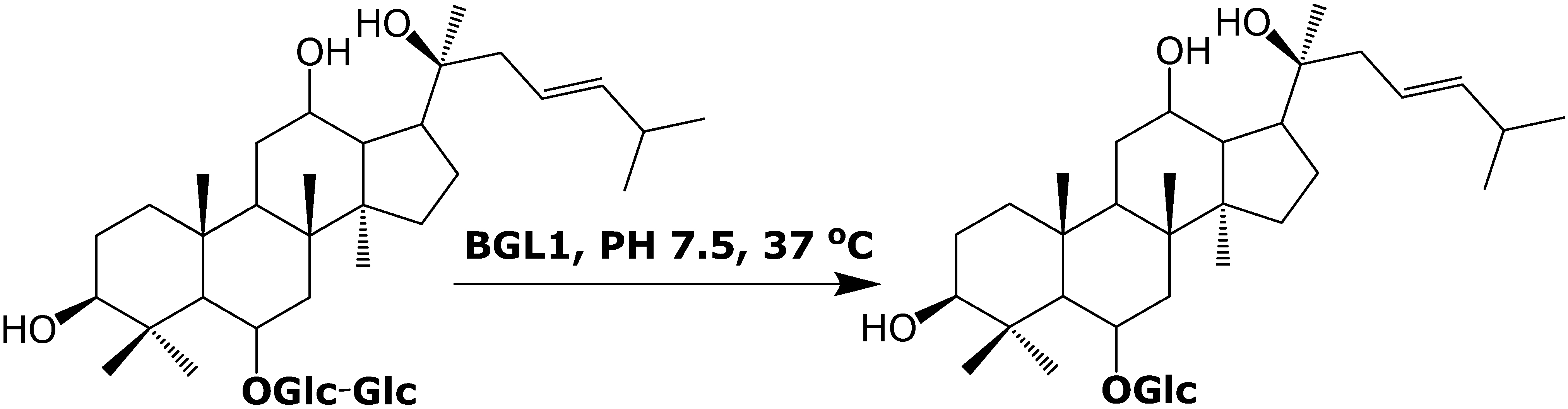
Results and Discussion
Expression and purification of BGL1 in Saccharomyces cerevisiae

In vitro biotransformation of ginsenoside Rf to Rh1

Conclusions
Experimental
General
Cloning and Expression of pRS-BGL1 in S. cerevisiae
Purification of recombinant BGL1 protein
Enzyme Activity
Acknowledgements
References and Notes
- Ligor, T.; Ludwiczuk, A.; Wolski, T.; Buszewski, B. Isolation and determination of ginsenosides in American ginseng leaves and root extracts by LC-MS. Anal. Bioanal. Chem. 2005, 383, 1098–1105. [Google Scholar] [CrossRef]
- Liu, W.; Xu, S.; Che, C. Anti-proliferative effect of ginseng saponins on human prostate cancer cell line. Life Sci. 2000, 67, 1297–130. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Park, J.; Lee, S. Ginsenoside-Rs4, a new type of ginseng saponin concurrently induces apoptosis and selectively elevates protein levels of p53 and p21WAF1 in human hepotoma SK-HEP-1 cells. Eur. J. Cancer 1999, 35, 507–511. [Google Scholar] [CrossRef]
- Lee, K.; Lee, Y.; Kim, S.; Park, J.; Lee, S. Ginsenoside-Rg5 suppresses cyclin E-dependent protein kinase activity via up-regulation of p21 Cip/WAF1 and down-regulating cyclin E in SKHEP-1 cells. Anticancer Res. 1997, 17, 1067–1072. [Google Scholar]
- Cho, W.; Chung, W.; Lee, S.; Leung, A.; Cheng, C.; Yue, K. Ginsenoside Re of Panax ginseng possesses significant antioxidant and antihyperlipidemic efficacies in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2006, 550, 173–179. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Lei, J. Studies on chemical components and their pharmacological activities of Panax ginseng root. Drug Discovery and Traditional Chinese Medicine: Science, Regulation, and Globalization; Kluwer Academic Publishers: University of Maryland, Maryland, USA, 2000; pp. 95–109. [Google Scholar]
- Akao, T.; Kida, H.; Kanaoka, M.; Hattori, M.; Kobashi, K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J. Pharm. Pharmacol. 1998, 50, 1155–1160. [Google Scholar] [CrossRef]
- Wakabayashi, C.; Hasegawa, H.; Murata, J.; Saiki, I. In vivo anti-metastatic action of ginseng proto-panaxadiol saponins is based on their intestinal metabolites after oral administration. Oncol. Res. 1997, 9, 411–417. [Google Scholar]
- Wakabayashi, C.; Hasegawa, H.; Murata, J. The expression of in vivo anti-metastatic effect of ginseng protopanaxadiol saponins is mediated by their intestinal metabolites after oral administration. J. Trad. Med. 1997, 14, 180–185. [Google Scholar]
- Hasegawa, H. Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with Fatty Acid. J. Pharmacol. Sci. 2004, 95, 153–157. [Google Scholar] [CrossRef]
- Lei, J.; Li, X.; Gong, X.; Zheng, Y. Isolation, synthesis and structures of cytotoxic ginsenoside. Molecules 2007, 12, 2140–2150. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, X.; Lee, K.; Zheng, Y.; Li, W.; Han, L.; Fang, Z.; Gu, L.; Sun, B.; Wang, C.; Sung, C. Optimization of ginsenosides hydrolyzing β-glucosidase production from Aspergillus niger using response surface methodology. Bio. Pharm. Bull. 2008, 31, 1870–1874. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ruan, C.-C.; Zhang, H.; Zhang, L.-X.; Liu, Z.; Sun, G.-Z.; Lei, J.; Qin, Y.-X.; Zheng, Y.-N.; Li, X.; Pan, H.-Y. Biotransformation of Ginsenoside Rf to Rh1 by Recombinant β-Glucosidase. Molecules 2009, 14, 2043-2048. https://doi.org/10.3390/molecules14062043
Ruan C-C, Zhang H, Zhang L-X, Liu Z, Sun G-Z, Lei J, Qin Y-X, Zheng Y-N, Li X, Pan H-Y. Biotransformation of Ginsenoside Rf to Rh1 by Recombinant β-Glucosidase. Molecules. 2009; 14(6):2043-2048. https://doi.org/10.3390/molecules14062043
Chicago/Turabian StyleRuan, Chang-Chun, Hao Zhang, Lian-Xue Zhang, Zhi Liu, Guang-Zhi Sun, Jun Lei, Yu-Xia Qin, Yi-Nan Zheng, Xiang Li, and Hong-Yu Pan. 2009. "Biotransformation of Ginsenoside Rf to Rh1 by Recombinant β-Glucosidase" Molecules 14, no. 6: 2043-2048. https://doi.org/10.3390/molecules14062043
APA StyleRuan, C. -C., Zhang, H., Zhang, L. -X., Liu, Z., Sun, G. -Z., Lei, J., Qin, Y. -X., Zheng, Y. -N., Li, X., & Pan, H. -Y. (2009). Biotransformation of Ginsenoside Rf to Rh1 by Recombinant β-Glucosidase. Molecules, 14(6), 2043-2048. https://doi.org/10.3390/molecules14062043



